Downregulation of STAT3 transcription factor reverses synaptotoxic phenotype of reactive astrocytes associated with prion diseases
- PMID: 40375298
- PMCID: PMC12080014
- DOI: 10.1186/s40478-025-02028-6
Downregulation of STAT3 transcription factor reverses synaptotoxic phenotype of reactive astrocytes associated with prion diseases
Abstract
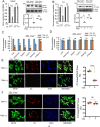
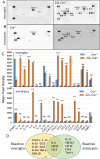
In neurodegenerative diseases, including prion diseases, astrocytes adopt reactive phenotypes that persist throughout disease progression. While astrocyte reactivity may initially serve as a protective response to prion infection, it transitions into a neurotoxic phenotype that disrupts homeostatic functions and exacerbates disease pathology. The transcription factor Stat3 has been recognized as a master regulator of astrocyte reactivity in neurodegenerative diseases, yet its role in prion disease-associated astrocyte reactive phenotypes remains unexplored. The current study addresses this gap by investigating the effects of Stat3 deletion in reactive astrocytes isolated from prion-infected mice. We demonstrate that Stat3 deletion mitigates the reactive astrocyte phenotype and alleviates their synaptotoxic effects. Stat3-dependent activation of astrocytes was reproduced by co-culturing naïve astrocytes with reactive microglia isolated from prion-infected animals or exposing them to microglia-conditioned media. A cytokine array profiling of 40 molecules revealed partially overlapping inflammatory signatures in reactive microglia and astrocytes, with IL-6 prominently upregulated in both cell types. Notably, IL-6 treatment elevated phosphorylated Stat3 levels in naïve astrocytes and triggered astrocyte reactivity. These findings indicate that the synaptotoxic phenotype of astrocytes in prion diseases can be sustained by reactive microglia and self-reinforced in a cell-autonomous manner. Our work highlights the pivotal role of Stat3 signaling in astrocyte activation and suggests that Stat3 inhibition may suppress the reactive phenotype of astrocytes associated with prion diseases.
Keywords: Interleukin 6; Neuroinflammation; Prion diseases; Prions; Reactive astrocytes; Reactive microglia; Stat3 transcription factor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore (Assurance Number: A32000-01; Protocol Numbers: 1120001 and 00000166-1). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Microglia-specific NF-κB signaling is a critical regulator of prion-induced glial inflammation and neuronal loss.PLoS Pathog. 2025 Jun 18;21(6):e1012582. doi: 10.1371/journal.ppat.1012582. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40532025 Free PMC article.
-
Non-cell autonomous astrocyte-mediated neuronal toxicity in prion diseases.Acta Neuropathol Commun. 2021 Feb 5;9(1):22. doi: 10.1186/s40478-021-01123-8. Acta Neuropathol Commun. 2021. PMID: 33546775 Free PMC article.
-
Modeling neuroinflammatory interactions between microglia and astrocytes in a human iPSC-based coculture platform.Cell Commun Signal. 2025 Jun 20;23(1):298. doi: 10.1186/s12964-025-02304-x. Cell Commun Signal. 2025. PMID: 40542355 Free PMC article.
-
Δ133p53α-mediated inhibition of astrocyte senescence and neurotoxicity as a possible therapeutic approach for neurodegenerative diseases.Neuroscience. 2025 Aug 6;580:54-61. doi: 10.1016/j.neuroscience.2025.06.031. Epub 2025 Jun 14. Neuroscience. 2025. PMID: 40523602 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Acioglu C, Li L, Elkabes S (2021) Contribution of astrocytes to neuropathology of neurodegenerative diseases. Brain Res:147291 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

