Mesenchymal stem/stromal cells as a therapeutic for sepsis: a review on where do we stand?
- PMID: 40375314
- PMCID: PMC12082945
- DOI: 10.1186/s13287-025-04371-w
Mesenchymal stem/stromal cells as a therapeutic for sepsis: a review on where do we stand?
Abstract
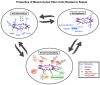
Sepsis is one of the leading causes of morbidity and mortality in the United States and Worldwide despite advances in quick recognition and early antibiotics, fluids, and vasopressors. Mesenchymal stem/stromal cells (MSCs) have gained attention as a biologic therapy given their unique anti-inflammatory, immunomodulatory, and anti-bacterial characteristics. MSCs have had success in treating a range of diseases, however limited clinical trials exist specifically for MSC use in sepsis. This article reviews the properties of MSCs that make them favorable for treating sepsis, as well as the current state of clinical trials. All clinical trials presented here demonstrated MSC safety, with a mixture of efficacy results and a heterogeneity of trial methods. Ultimately, MSCs are a promising novel therapeutic for sepsis, however a consensus in cell source, dosage, preparation, and delivery needs to be further investigated for MSCs to transition from bench to bedside and become a true therapeutic for sepsis.
Keywords: Clinical trials; MSC; Mesenchymal stem cells; Sepsis; Septic shock.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: n/a. Consent for publication: All authors have given consent for publication in Stem Cell Research & Therapy. Competing interests: JMH is the Chief Scientific Officer, a compensated consultant and advisory board member for Longeveron, and holds equity in Longeveron. JMH is also the co-inventor of intellectual property licensed to Longeveron. The University of Miami also stands to gain royalties from the commercialization of the IP. JMH and CPB have a patent for monitoring efficacy of mesenchymal stem cell therapy. The other authors declare no competing interests.
Figures
Similar articles
-
Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential.Expert Rev Respir Med. 2021 Mar;15(3):301-324. doi: 10.1080/17476348.2021.1848555. Epub 2020 Nov 26. Expert Rev Respir Med. 2021. PMID: 33172313 Review.
-
Immunophenotypic characterization and therapeutics effects of human bone marrow- and umbilical cord-derived mesenchymal stromal cells in an experimental model of sepsis.Exp Cell Res. 2021 Feb 15;399(2):112473. doi: 10.1016/j.yexcr.2021.112473. Epub 2021 Jan 8. Exp Cell Res. 2021. PMID: 33428902
-
Mesenchymal stem cells preconditioned by staphylococcal enterotoxin B enhance survival and bacterial clearance in murine sepsis model.Cytotherapy. 2019 Jan;21(1):41-53. doi: 10.1016/j.jcyt.2018.11.002. Epub 2018 Nov 23. Cytotherapy. 2019. PMID: 30477894
-
Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cell Therapy in Preclinical Models of Sepsis: A Systematic Review and Meta-analysis.Stem Cells Transl Med. 2024 Apr 15;13(4):346-361. doi: 10.1093/stcltm/szae003. Stem Cells Transl Med. 2024. PMID: 38381583 Free PMC article.
-
Bone marrow vs Wharton's jelly mesenchymal stem cells in experimental sepsis: a comparative study.Stem Cell Res Ther. 2019 Jun 27;10(1):192. doi: 10.1186/s13287-019-1295-9. Stem Cell Res Ther. 2019. PMID: 31248453 Free PMC article.
References
-
- Evans L, Rhodes A, Alhazzani W et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11). - PubMed
-
- Premer C, Schulman IH, Jackson JS. The role of mesenchymal stem/stromal cells in the acute clinical setting. Am J Emerg Med. 2021;46:572–578. 10.1016/j.ajem.2020.11.035 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

