Balancing Chemical and Supramolecular Stability in OEGylated Supramolecular Polymers for Systemic Drug Delivery
- PMID: 40375654
- PMCID: PMC12372228
- DOI: 10.1021/jacs.5c03253
Balancing Chemical and Supramolecular Stability in OEGylated Supramolecular Polymers for Systemic Drug Delivery
Abstract
The chemical conjugation of poly(ethylene glycol) (PEG) to therapeutic agents, known as PEGylation, is a well-established strategy for enhancing drug solubility, chemical stability, and pharmacokinetics. Here, we report on a class of supramolecular polymeric prodrugs by utilizing oligo(ethylene glycol) (OEG) to modify the hydrophobic anticancer drug camptothecin (CPT). These OEGylated prodrugs, despite their low molecular weight, spontaneously self-assemble into therapeutic supramolecular polymers (SPs) with a tubular morphology, featuring a dense OEG coating on the surface. By designing biodegradable linkers with varying chemical stabilities, we investigated how the release kinetics of CPT influence the in vitro and in vivo performance of these SPs. Our findings demonstrate that self-assembling prodrugs (SAPDs) with a self-immolative disulfanyl-ethyl carbonate (etcSS) linker exhibit a faster drug release rate than those with a reducible disulfanyl butyrate (buSS) linker, leading to higher potency and significantly improved antitumor efficacy. Notably, two stable tubular SPs, Tubustecan (TT) 1E and TT 7E, outperformed irinotecan─a clinically approved CPT prodrug─in a colon cancer model, achieving enhanced tumor growth inhibition and prolonged animal survival. These results highlight the potential of supramolecular OEGylation as an important strategy for engineering drug-based supramolecular polymers and underscore the critical role of chemical stability vs supramolecular stability in optimizing supramolecular prodrug design.
Figures

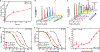
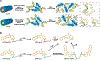




Similar articles
-
Structure-guided dual modification prodrug strategy for optimizing camptothecin delivery in pancreatic cancer chemotherapy.Int J Pharm. 2025 Sep 15;682:125908. doi: 10.1016/j.ijpharm.2025.125908. Epub 2025 Jun 27. Int J Pharm. 2025. PMID: 40582523
-
Supramolecular assembly of isomeric SN-38 prodrugs regulated by conjugation sites.J Mater Chem B. 2024 Jun 27;12(25):6146-6154. doi: 10.1039/d4tb00717d. J Mater Chem B. 2024. PMID: 38842181
-
Development and in vitro evaluation of lipodisks optimized for co-delivery of chemotherapeutic drugs and membranolytic anticancer peptides.J Colloid Interface Sci. 2025 Dec;699(Pt 1):138173. doi: 10.1016/j.jcis.2025.138173. Epub 2025 Jun 16. J Colloid Interface Sci. 2025. PMID: 40527146
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Webber MJ; Appel EA; Meijer EW; Langer R, Supramolecular biomaterials. Nat Mater 2016, 15 (1), 13–26. - PubMed
-
- Wang H; Su H; Xu T; Cui HG, Utilizing the Hofmeister Effect to Induce Hydrogelation of Nonionic Supramolecular Polymers into a Therapeutic Depot. Angew Chem Int Edit 2023, 62 (43), e202306652. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

