The Human Bone Marrow May Offer an IL-15-Dependent Survival Niche for EOMES+ Tr1-Like Cells
- PMID: 40375822
- PMCID: PMC12082382
- DOI: 10.1002/eji.202451644
The Human Bone Marrow May Offer an IL-15-Dependent Survival Niche for EOMES+ Tr1-Like Cells
Abstract
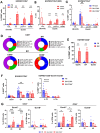
Maintenance of memory T-cells in the bone marrow and systemically depends on the homeostatic cytokines IL-7 and IL-15. An immunological memory may also exist for regulatory T-cells. EOMES+type-1 regulatory (Tr1)-like cells have a rapid in vivo turnover, but whether they are short-lived effector cells or are maintained long-term has not been investigated. EOMES+Tr1-like cells expressing GzmK were enriched among CD69+Ki67-T-cells in the bone marrow of healthy donors, suggesting that they became quiescent and bone marrow-resident. Conversely, CD4+GzmB+ effector T-cells were excluded from the bone marrow-resident fraction. The dichotomy between GzmK+ and GzmB+T-cells was observed both in healthy individuals and in multiple sclerosis patients, and also among CD8+T-cells. Intriguingly, bone marrow-resident CD4+ memory T-cells expressed increased levels of IL-7Rα, while EOMES+Tr1-like cells were consistently IL-7Rαlo. However, EOMES+Tr1-like cells expressed the IL-2/15Rβ chain, and the latter was induced upon forced expression of EOMES in primary human CD4+ T-cells. Finally, IL-15 rescued EOMES+Tr1-enriched populations from death by neglect but was not required for CD4+ memory T-cell survival. These findings suggest that the bone marrow may provide a survival niche for EOMES+Tr1-like cells. The different IL-7 and IL-15 receptor expression patterns of CD4+ memory T-cells and EOMES+Tr1-like cells suggest furthermore that they compete for different homeostatic niches.
Keywords: CD69; bone marrow; memory T lymphocytes; regulatory T‐cells.
© 2025 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Sprent J. and Surh C. D., “T Cell Memory,” Annual Review of Immunology 20 (2002): 551–579. - PubMed
-
- Manz R. A., Thiel A., and Radbruch A., “Lifetime of Plasma Cells in the Bone Marrow [letter],” Nature 388 (1997): 133–134. - PubMed
-
- Zhang X., Sun S., Hwang I., Tough D. F., and Sprent J., “Potent and Selective Stimulation of Memory‐phenotype CD8+ T Cells in Vivo by IL‐15,” Immunity 8 (1998): 591–599. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

