An Update of the Appropriate Sequencing for Salvage Therapies in Multiple Myeloma
- PMID: 40375908
- PMCID: PMC12081049
- DOI: 10.4084/MJHID.2025.043
An Update of the Appropriate Sequencing for Salvage Therapies in Multiple Myeloma
Abstract
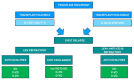
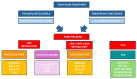
Current treatment strategies have led to unpredictable improvements in the management of multiple myeloma (MM) over time. However, resistance to therapy, particularly regarding lenalidomide refractoriness and, more recently, daratumumab and lenalidomide refractoriness, even in the first-line setting, has become an increasingly significant issue in recent years. This resistance complicates the identification of the optimal treatment algorithm for patients with relapsed/refractory MM, particularly at first relapse. In this review, we focus on current strategies for MM patients progressing on or after lenalidomide-based and daratumumab-lenalidomide-based regimens. The forthcoming availability of next-generation immunotherapies, along with a deeper understanding of resistance mechanisms, is highly anticipated. Meanwhile, based on promising results from recent studies, the approval of novel drugs to expand the current therapeutic armamentarium against MM is bringing us closer to the goal of making a potential cure for the disease much more achievable in the hopefully near future.
Keywords: Bispecific antibody; CAR-T cells; Immunotherapy; Multiple myeloma; Refractory Disease.
Conflict of interest statement
Competing interests: The authors declare no conflict of Interest.
Figures
References
-
- Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Blade J, Mateos MV, Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H, Spencer A, Hou J, Chng WJ, Usmani SZ, Zamagni E, Shimizu K, Jagannath S, Johnsen HE, Terpos E, Reiman A, Kyle RA, Sonneveld P, Richardson PG, McCarthy P, Ludwig H, Chen W, Cavo M, Harousseau JL, Lentzsch S, Hillengass J, Palumbo A, Orfao A, Rajkumar SV, Miguel JS, Avet-Loiseau H. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46. doi: 10.1016/S1470-2045(16)30206-6. - DOI - PubMed
-
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi NC, San Miguel J International Myeloma Workshop Consensus P. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5. doi: 10.1182/blood-2010-10-299487. - DOI - PMC - PubMed
-
- Moreau P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, Hajek R, Spicka I, Baker R, Kim K, Martinez G, Min CK, Pour L, Leleu X, Oriol A, Koh Y, Suzuki K, Risse ML, Asset G, Mace S, Martin T group Is. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397:2361–71. doi: 10.1016/S0140-6736(21)00592-4. - DOI - PubMed
-
- Martin T, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, Hajek R, Spicka I, Baker R, Kim K, Martinez G, Min CK, Pour L, Leleu X, Oriol A, Koh Y, Suzuki K, Casca F, Mace S, Risse ML, Moreau P. Isatuximab, carfilzomib, and dexamethasone in patients with relapsed multiple myeloma: updated results from IKEMA, a randomized Phase 3 study. Blood Cancer J. 2023;13:72. doi: 10.1038/s41408-023-00797-8. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources