Bridging the gap: enhancing blood regulatory functions in African contexts through comparative analysis
- PMID: 40375930
- PMCID: PMC12078219
- DOI: 10.3389/fmed.2025.1519719
Bridging the gap: enhancing blood regulatory functions in African contexts through comparative analysis
Abstract
Introduction: Independent assessments of blood regulatory systems, facilitated by tools such as the WHO's Global Benchmarking Tool (GBT) plus Blood expedites development of National Regulatory Authorites (NRAs) and thus promotes increased access to safe, effective, and quality blood, blood components, and products. The aim of this study was to assess and compare the status of implementation and performance of the regulatory functions for registration and marketing authorization as well as the system for approval of blood, blood components and plasma for fractionation or processes.
Methods: We did this by conducting assisted self-benchmarking in 12 African countries using the GBT plus Blood (registration and marketing authorization function, 34 sub-indicators and approval of blood, blood components, and plasma for fractionation or processes function, 24 sub-indicators). Comparative assessments of WHO-designated maturity level 3 (ML3) NRAs for medicines and vaccines against non-designated NRAs were made.
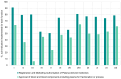
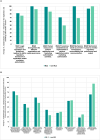
Results: The percentage of implemented sub-indicators was higher for the registration and marketing authorization function with an average implementation score of 73% (range: 51%-92%) compared to the approval of blood, blood components, and plasma for fractionation or processes function which had an average implementation score of 45% (range: 6%-65%). The comparison of group averages for the ML3-designated NRAs against the non-designated NRAs revealed a higher score 91% (range: 71%-100%) for ML3-designated NRAs as opposed to a lower score of 71% (range: 49%-100%) for the non-designated NRAs for the registration and marketing authorization function. This pattern, however, was not observed for the comparison of group averages for the approval of blood, blood components, and plasma for fractionation or processes function where the ML3-designated NRAs scored 47% (range 19%-72%) against 46% (range 23%-88%) for the non-ML3-designated NRAs.
Conclusion: Most of the NRAs excelled in implementing sub-indicators for the registration and marketing authorization (of plasma-derived medicines) function. All NRAs exhibited notable flaws in regulating blood, blood components, plasma for fraction, and approval of processes, indicating nascent regulatory frameworks. This study highlights the urgent need for WHO and African countries to prioritize formal benchmarking of NRAs using the GBT plus Blood to enhance their regulatory capacities in blood and blood product regulation.
Keywords: GHPP BloodTrain; Sub-Saharan African countries; availability of safe blood; blood and blood products; global benchmarking tool.
Copyright © 2025 Samukange, Kluempers, Kafere, Heinrich, Atemnkeng, Khadem Broojerdi, Tirane, Nkansah, Maboko, Nhukarume, Mutoti, Aineplan, Gardasdottir, Mantel-Teeuwisse and Reinhardt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Epstein J, Seitz R, Dhingra N, Ganz PR, Gharehbaghian A, Spindel R, et al. Role of regulatory agencies. Biologicals. (2009) 37:94–102. Available online at: https://www.sciencedirect.com/science/article/abs/pii/S1045105609000050?... - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

