Engineering extracellular matrix-based hydrogels for intervertebral disc regeneration
- PMID: 40375978
- PMCID: PMC12078266
- DOI: 10.3389/fbioe.2025.1601154
Engineering extracellular matrix-based hydrogels for intervertebral disc regeneration
Abstract
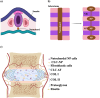
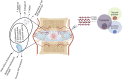
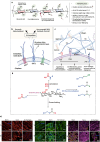
Lower back pain (LBP) is a major health concern, especially in older adults. A key aetiological factor is intervertebral disc (IVD) degeneration. It is mediated by dysregulation of extracellular matrix (ECM) and inflammation. In recent years, regenerative therapies have garnered attention for their potential to restore disc function by addressing the underlying biological alterations within the IVD. This review focuses on the comprehensive understanding of the anatomy and physiology of the IVD, highlighting its life cycle from embryonic development, and maturation to degenerative phenotype. We describe current treatments for managing LBP caused by IVD degeneration. This review emphasizes on the recent advancements in hydrogel engineering, highlighting natural, synthetic, and composite hydrogels and their application in ECM-targeted regenerative therapy for IVD degeneration. By exploring innovations in hydrogel technology, including improvements in crosslinking techniques and controlled degradation rates-we discuss how these materials could enhance IVD regeneration and potentially be used for the management of LBP. With their enhanced biomimicry, hydrogel-based ECM mimics offer a promising pathway for developing effective, durable therapies that address the root causes of disc degeneration, providing new hope for individuals living with chronic LBP.
Keywords: biomaterials; extracellular matrix; hydrogel; intervertebral disc degeneration; lower back pain.
Copyright © 2025 Kmail, Razak and Mohd Isa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Adoungotchodo A., Epure L. M., Mwale F., Lerouge S. (2021). Chitosan-based hydrogels supplemented with gelatine and Link N enhance extracellular matrix deposition by encapsulated cells in a degenerative intervertebral disc environment. Eur. Cell Mater 41, 471–484. 10.22203/ecm.v041a30 - DOI - PubMed
-
- Anjankar S. D., Poornima S., Raju S., Jaleel M., Bhiladvala D., Hasan Q. (2015). Degenerated intervertebral disc prolapse and its association of collagen I alpha 1 Spl gene polymorphism: a preliminary case control study of Indian population. Indian J. Orthop. 49 (6), 589–594. 10.4103/0019-5413.168765 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

