Anti-tumor effect and immune-related mechanism study of compound aluminum sulfate injection in transplanted tumor-bearing mice
- PMID: 40375992
- PMCID: PMC12078244
- DOI: 10.3389/fimmu.2025.1583275
Anti-tumor effect and immune-related mechanism study of compound aluminum sulfate injection in transplanted tumor-bearing mice
Abstract
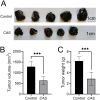
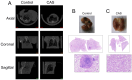
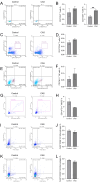
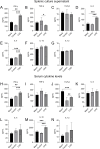
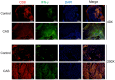
This study investigates the antitumor and immunomodulatory effects of compound aluminum sulfate (CAS) solution in murine melanoma models. Using syngeneic B16-F10 and B16-OVA tumor models, we demonstrate that intratumoral CAS injection significantly inhibits primary tumor growth and lung metastasis. Flow cytometry analysis reveals that CAS treatment increases splenic populations of CD3+CD8+ cytotoxic T cells, CD3+CD44+ memory T cells, and NK cells, while enhancing CD8+ T cell infiltration in tumor tissue. ELISA results show elevated levels of pro-inflammatory cytokines (IFN-γ, TNF-α, and IL-2) in splenic culture supernatants and serum following CAS administration. Immunofluorescence staining confirms increased expression of CD8 and IFN-γ proteins in tumor tissues of CAS-treated mice. Results indicate that CAS exerts its antitumor effects through direct cytotoxicity and by modulating both systemic and local immune responses. The dual action of CAS, which combines tumor necrosis with immunostimulation, positions it as a promising therapeutic agent for cancer treatment. This study offers valuable insights into the mechanisms underlying CAS's action and underscores its potential clinical applications in oncology.
Keywords: anti-tumor effect; compound aluminum sulfate injection; immunomodulator; melanoma; metastasis.
Copyright © 2025 Shi, Xia, Huang, Chen, Yin, Xin and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Zhao G, Yang F, Yang Q, Yang Y, Xu A, Chen Y, et al. Efficacy of compound aluminum sulfate injection as a monotherapeutic regimen in non-muscle invasive bladder cancer patients: a retrospective single-arm cohort study. Transl Androl Urol. (2022) 11:1292–303. doi: 10.21037/tau-22-483 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

