Nrf2-dependent effects of CDDO-Me on bactericidal activity in macrophage infection models
- PMID: 40376003
- PMCID: PMC12078304
- DOI: 10.3389/fimmu.2025.1574776
Nrf2-dependent effects of CDDO-Me on bactericidal activity in macrophage infection models
Abstract
Introduction: Diabetes and chronic kidney disease (CKD) increase susceptibility to bacterial infections, particularly Staphylococcus aureus, which is associated with highmortality in CKD patients. Dysregulated macrophage activity and excessive oxidative stress exacerbate immune dysfunction and inflammation in these conditions. Nrf2 (nuclear factor erythroid 2-related factor 2) is a key regulator of antioxidant defenses and macrophage function. CDDO-Me, a synthetic triterpenoid, activates Nrf2, providing antioxidant and anti-inflammatoryeffects. However, its precise role in modulating macrophage activity, polarization, and bacterial clearance remains unclear.
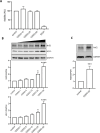
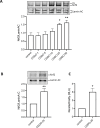
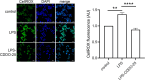
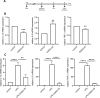
Methods: The effects of CDDO-Me on macrophage function were evaluated in vitro (THP-1 and RAW 264.7 macrophages) and an in vivo Nrf2 knockout mouse model. Nrf2 activation was assessed via Western blot and luciferase reporter assays, oxidative stress was measured using CellROX reagent, and inflammatory responses were quantified by RT-qPCR. Intracellular S. aureus survival and macrophage polarization markers were analyzed to investigate the role of CDDO-Me in enhancing bactericidal activity.
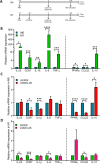
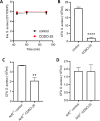
Results: Our results showed that CDDO-Me activated the Nrf2 signaling pathway, reducing oxidative stress and inflammation in macrophages by downregulating pro-inflammatory cytokines (IL-1β, TNF-α). It modulated macrophage polarization, decreasing M1 and M2 marker expression, and significantly enhanced bactericidal activity against S. aureus. These effects were Nrf2-dependent, as demonstrated in knockout models.
Conclusion: The ability of CDDO-Me to regulate oxidative stress, inflammation, and bacterial clearance underscores its therapeutic potential for managing inflammatory and infectious diseases indiabetes and CKD.
Keywords: CDDO-Me; Nrf2 knockout mice; Staphylococcus aureus; bactericidal activity; bronchoalveolar lavage; inflammation; macrophages; oxidative stress.
Copyright © 2025 Deramaudt, Chehaitly and Bonay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

