Pilot evaluation of a novel, automated ergonomics assessment tool
- PMID: 40376029
- PMCID: PMC12080516
- DOI: 10.1055/a-2568-9610
Pilot evaluation of a novel, automated ergonomics assessment tool
Erratum in
-
Correction: Pilot evaluation of a novel, automated ergonomics assessment tool.Endosc Int Open. 2025 Jun 4;13:a26258430. doi: 10.1055/a-2625-8430. eCollection 2025. Endosc Int Open. 2025. PMID: 40932822 Free PMC article.
Abstract
Background and study aims: Gastroenterologists are prone to endoscopy-related musculoskeletal injuries (ERI). Current interventions lack real-time monitoring and feedback. ErgoGenius, a novel artificial intelligence computer-vision tool, addresses this gap by providing continuous posture assessment and feedback without wearable motion trackers. The aim of this study was to determine the feasibility of ErgoGenius, its accuracy compared with human appraisers, and its ability to detect abnormal posture.
Methods: The study was conducted at two large academic centers. The Rapid Entire Body Assessment (REBA) score was used as a surrogate for ergonomic performance and risk of injury. Ten endoscopists of varying gender, height, and weight were recorded performing endoscopic tasks in optimal vs. lowered bed positions. Videos were analyzed by ErgoGenius. A paired t -test was used to compare REBA scores between bed positions.
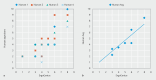
Results: ErgoGenius was successfully deployed in a controlled endoscopy setting. ErgoGenius achieved perfect internal agreement (rho = 1) and closely correlated with human appraisers (rho = 0.987). Average REBA scores were notably higher in the lowered bed position (mean 4.64) compared with the optimal position (mean 2.55), ( P = 0.006).
Conclusions: ErgoGenius was successfully deployed to detect abnormal postures related to changes in bed position and quantify ERI risk. It performed at par with human appraisers. This tool shows promise in enhancing ergonomic practices among gastroenterologists and trainees, potentially leading to better health outcomes and reduced injury.
Keywords: Endoscopy Lower GI Tract; Endoscopy Small Bowel; Endoscopy Upper GI Tract.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
Conflict of Interest Michael Kochman has served as a consultant for Applied Clinical Intelligence (adjudication committee), Boston Scientific Corporation (tissue acquisition), Castle Biosciences (Barretts diagnostics), Dark Canyon Laboratories (colonoscopy prep), Endiatx (steerable capsule), Olympus (scientific advisory panel), and Virgo Systems (artificial intelligence) and holds equity in the American Gastroenterological Association and Varia Ventures GI Opportunity Fund (venture capital), Dark Canyon Labs (colonoscopy preparation), Endiatx (steerable capsule), Endosound (endoscopic ultrasound device), Varia Ventures (venture capital), and Virgo. None of the other authors have any relevant disclosures.
Figures

References
-
- Cohen LB, Wecsler JS, Gaetano JN et al. Endoscopic sedation in the United States: Results from a Nationwide Survey. Am J Gastroenterol. 2006;101:967–974. - PubMed
-
- ASGE Technology Committee . Pedrosa MC, Farraye FA et al. Minimizing occupational hazards in endoscopy: personal protective equipment, radiation safety, and ergonomics. Gastrointest Endosc. 2010;72:227–235. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

