Drug-induced cardiac arrest: a pharmacovigilance study from 2004-2024 based on FAERS database
- PMID: 40376150
- PMCID: PMC12078221
- DOI: 10.3389/fcvm.2025.1498700
Drug-induced cardiac arrest: a pharmacovigilance study from 2004-2024 based on FAERS database
Abstract
Objective: Utilizing the FDA Adverse Event Reporting System (FAERS) database, this study conducts signal detection for drugs associated with cardiac arrest (CA), aiming to optimize clinical decision-making and ensure safer drug usage.
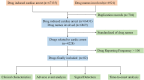
Methods: Adverse event reports related to CA from the first quarter of 2004 to the second quarter of 2024 were extracted from the FAERS database. Signal detection was conducted using the reporting odds ratio (ROR) and proportional reporting ratio (PRR) to identify drugs associated with an increased risk of CA.
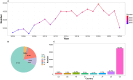
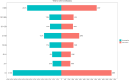
Results: A total of 66,431 reports were analyzed, comprising 34,508 males (51.9%) and 31,923 females (48.1%). The majority of cases (71.8%) were reported by healthcare professionals, with adults (≥18 years old) representing the predominant group. Clinical outcomes showed that 67.2% of cases resulted in death. Out of 82 drugs with over 100 CA-related reports, 43 displayed positive signals. The top five drugs identified by ROR were: carisoprodol [ROR (95% CI): 34.13 (29.62-39.32)], sugammadex [ROR (95% CI): 26.93 (22.56-32.16)], regadenoson [ROR (95% CI): 20.00 (17.69-22.60)], alprazolam [ROR (95% CI): 12.82 (12.19-13.48)], and propofol [ROR (95% CI): 11.93 (10.61-13.41)]. In the system drug signal detection, musculo-skeletal system drugs ranked highest [ROR (95% CI): 30.99 (27.74-34.62)], followed by alimentary tract and metabolism drugs [ROR (95% CI): 4.75 (4.59-4.92)], nervous system drugs [ROR (95% CI): 4.51 (4.4-4.61)], anti-infective drugs [ROR (95% CI): 4.13 (3.74-4.57)], cardiovascular drugs [ROR (95% CI): 3.89 (3.78-4.01)], and antineoplastic and immunomodulating agents [ROR (95% CI): 2.16 (2.13-2.2)].
Conclusion: This study identifies over 40 drugs potentially associated with an elevated risk of CA based on FAERS data. Healthcare professionals should be particularly vigilant when prescribing these drugs, especially to patients with a history of heart disease, and ensure rigorous monitoring of their cardiac health.
Keywords: FAERS; FDA; adverse events; cardiac arrest; pharmacovigilance.
© 2025 Ren, Huang, Zhang, Liu, Yan and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Habacha S, Mghaieth Zghal F, Boudiche S, Fathallah I, Blel Y, Aloui H, et al. Toxin-induced cardiac arrest: frequency, causative agents, management and hospital outcome. Tunis Med. (2020) 98(2):123–30. - PubMed
-
- Hannen LEM, Toprak B, Weimann J, Mahmoodi B, Fluschnik N, Schrage B, et al. Clinical characteristics, causes and predictors of outcomes in patients with in-hospital cardiac arrest: results from the SURVIVE-ARREST study. Clin Res Cardiol. (2023) 112(2):258–69. 10.1007/s00392-022-02084-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

