Sono-Gas-Mediated Precise Stiffness Remodeling for Triple-Negative Breast Cancer Mechanical Immunotherapy
- PMID: 40376200
- PMCID: PMC12078941
- DOI: 10.34133/bmr.0207
Sono-Gas-Mediated Precise Stiffness Remodeling for Triple-Negative Breast Cancer Mechanical Immunotherapy
Abstract
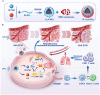
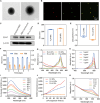
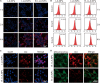
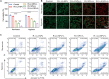
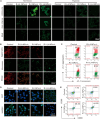
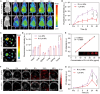
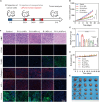
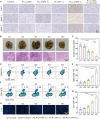
Triple-negative breast cancer (TNBC) is a highly invasive cancer, and its poor therapeutic outcomes are often associated with the mechanical properties of the tumor microenvironment, which is characterized by altered extracellular matrix (ECM) flexibility and increased stiffness. Herein, a mechanical immunomodulator, namely, red blood cell membrane-IR780-L-arginine nanoparticles (R-I-LA NPs), was designed to precisely regulate the stiffness of the ECM for mechanical immunotherapy of TNBC. In tumor cells, the low-intensity focused ultrasound activates R-I-LA NPs to produce reactive nitrogen species, which damages tumor cells and remodels the stiffness of ECM. Meanwhile, the softened ECM can normalize the tumor vasculature to alleviate hypoxia and increase the production of reactive oxygen species, thereby enhancing the efficacy of sonodynamic therapy and stimulating immunogenic cell death. Additionally, R-I-LA NPs stimulate the immune system and suppress pulmonary metastasis. Overall, this study offers a distinctive "sono-gas-mediated mechanical immunity" strategy for ECM regulation, potentially overcoming current TNBC therapy limitations.
Copyright © 2025 Yaqin Hu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381: Article e071674. - PubMed
-
- Lakshmanan VK, Jindal S, Packirisamy G, Ojha S, Lian S, Kaushik A, Alzarooni A, Metwally YAF, Thyagarajan SP, Do Jung Y, et al. . Nanomedicine-based cancer immunotherapy: Recent trends and future perspectives. Cancer Gene Ther. 2021;28(9):911–923. - PubMed
-
- Nguyen HM, Paulishak W, Oladejo M, Wood L. Dynamic tumor microenvironment, molecular heterogeneity, and distinct immunologic portrait of triple-negative breast cancer: An impact on classification and treatment approaches. Breast Cancer. 2023;30(2):167–186. - PubMed
-
- Wang Y, Goliwas KF, Severino PE, Hough KP, Van Vessem D, Wang H, Tousif S, Koomullil RP, Frost AR, Ponnazhagan S, et al. . Mechanical strain induces phenotypic changes in breast cancer cells and promotes immunosuppression in the tumor microenvironment. Lab Investig. 2020;100(12):1503–1516. - PMC - PubMed
LinkOut - more resources
Full Text Sources

