CKD-associated pruritus in haemodialysis: a road map for diagnosis and treatment
- PMID: 40376308
- PMCID: PMC12080227
- DOI: 10.1093/ckj/sfaf096
CKD-associated pruritus in haemodialysis: a road map for diagnosis and treatment
Abstract
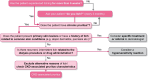
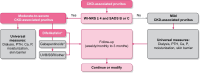
Of the wide range of symptoms affecting patients with chronic kidney disease (CKD) on haemodialysis, CKD-associated pruritus is one of the most common and burdensome, occurring at moderate-to-severe intensity in 31%-40% of patients, significantly impacting multiple aspects of quality of life, and associated with increased healthcare utilization. Despite the distressing nature of this symptom, clinicians frequently underestimate its prevalence and it is under-reported by patients who may be unaware of the availability of effective treatment options. The identification and management of CKD-associated pruritus should form an essential aspect of patient-centred care; however, patients with CKD may have multiple causes of chronic itch including those of dermatological, systemic, neuropathic and psychogenic origin, and CKD-associated pruritus must be distinguished from these. Together with its highly variable presentation in patients on haemodialysis, the range of potential causes of itch makes differential diagnosis of CKD-associated pruritus challenging. The presence of bilaterally symmetrical and non-dermatomally distributed itching, commonly affecting the back, limbs, chest and head is characteristic of CKD-associated pruritus, although approximately 50% of patients report generalized pruritus. Secondary skin lesions (including excoriation, crusts, impetigo, lichenifications and prurigo also seen in dermatological conditions) may or may not be observed, and xerosis (dry skin) that may exacerbate itching is common. Here, we provide a pragmatic approach to the identification and differential diagnosis of chronic itching in CKD-associated pruritus with the aim of supporting the effective management of this highly distressing symptom in clinical practice.
Keywords: CKD-aP; chronic kidney disease; diagnosis; haemodialysis; pruritus.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
J.L. reported receiving consulting fees, speaker fees, travel support from CSL Vifor and advisory board participation for CSL Vifor. A.L. reported receiving consulting fees from CSL Vifor, Bayer Health, AstraZeneca; speaker fees from CSL Vifor, AstraZeneca, Baxter; and travel support from CSL Vifor. S.S. reported grants/research funding from Almirall S.A., Sanofi Genzyme Corporation, Galderma SA, Trevi Therapeutics Inc., Beiersdorf AG; consulting fees from AbbVie Deutschland GmbH & Co., Amgen Inc., Bellus Health Cough Inc., Bristol-Meyers Squibb Company, Clexio Biosiences Ltd, Galderma S.A., Klirna Biotech Inc., Omnicuris Healthcare Private Ltd, Pfizer Inc., P.G. Unna Academy, Sanofi-Aventis Deutschland GmbH, Sanofi-Aventis R & D, Sanofi Genzyme Corporation, Vifor Pharma Deutschland GmbH; speaker fees from Beiersdorf AG, CRM GmbH, FomF GmbH, Galderma Laboratorium GmbH, Galderma SA, L'Oréal, Novartis Pharma GmbH, Pfizer Pharma GmbH, P.G. Unna Akademie e.V., Sanofi-Aventis (Schweiz) AG, Sanofi-Aventis Deutschland GmbH, Sanofi B.V., Sanofi Genzyme Europe B.V., Sanofi Genzyme Corporation, Sanofi Hong Kong Ltd, Sanofi OY, Streamed-Up! GmbH, TouchIME, UCB Pharma GmbH, Vifor Pharma Deutschland GmbH, WebMD Global LLC; travel support from AbbVie Deutschland GmbH & Co., Attovia Therapeutics Inc., Trevi Therapeutics Inc., Galderma Laboratorium GmbH, Sanofi-Aventis Deutschland GmbH, Lilly Deutschland GmbH, Pfizer Pharma GmbH, Sanofi Genzyme Corporation, Vifor Pharma Deutschland GmbH; and advisory board participation for Amgen Europe GmbH, Focus-Insight Healthtech Group Co. Ltd, Lilly Deutschland GmbH, Almirall Hermal GmbH, AbbVie Deutschland GmbH Co. KG, Almirall S.A., Celldex Therapeutics Inc., Galderma R & D, LLC, Galderma Laboratorium GmbH, Galderma SA, Grünenthal GmbH, Lilly Deutschland GmbH, Regeneron Pharmaceuticals Inc., Sanofi Aventis Deutschland GmbH, Sanofi Genzyme Corporation and Vifor Pharma Deutschland GmbH. E.S.-A. reported no conflicts of interest. F.A. reported receiving speaker fees from AstraZeneca and advisory board participation for CSL Vifor and Sanofi. G.Y. reported grants/research funding from Eli Lilly LEO Pharma, Novartis, Pfizer, Galderma, Escient, Sanofi Regeneron, Sanofi Celldex, Pfizer, Regeneron Pharmaceuticals Inc., Sanofi, Galderma, Amgen, Clexio, AbbVie; consulting fees from AbbVie, Arcutis, Almiral, Amgen, Celldex, CSL Vifor, Escient Health, Eli Lilly, Galderma, GSK, LEO Pharma, Merck, Novartis, Pfizer, Pierre Fabre, Regeneron Pharmaceuticals Inc., Sanofi; advisory board participation for Kamari; and speaker fees from Piere Fabre, Regeneron Pharmaceuticals Inc., Merck. G.Y. is the founder and past president of the International Forum for the Study of Itch (IFSI) and a member of its board. G.Y. served as Chair of the Scientific Board for the National Eczema Association (NEA), is a member of the Scientific Board of The National Psoriasis Foundation (NPF) and is a counsellor for the International Eczema Council (IEC).
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

