Nanotechnology meets medicine: applications of atomic force microscopy in disease
- PMID: 40376402
- PMCID: PMC12075069
- DOI: 10.1007/s12551-025-01306-w
Nanotechnology meets medicine: applications of atomic force microscopy in disease
Abstract
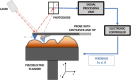
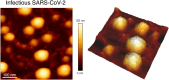
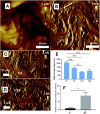
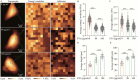
Atomic force microscopy (AFM) is a scanning imaging technique able to work at the nanoscale. It uses a cantilever with a tip to move across samples' surface and a laser to measure the cantilever bending, enabling the assessment of interaction forces between tip and sample and creating a three-dimensional visual representation of its surface. AFM has been gaining notoriety in the biomedical field due to its high-resolution images, as well as due to its ability to measure the inter- and intramolecular interaction forces involved in the pathophysiology of many diseases. Here, we highlight some of the current applications of AFM in the biomedical field. First, a brief overview of the AFM technique is presented. This theoretical framework is then used to link AFM to its novel translational applications, handling broad clinical questions in different areas, such as infectious diseases, cardiovascular diseases, cancer, and neurodegenerative diseases. Morphological and nanomechanical characteristics such as cell height, volume, stiffness, and adhesion forces may serve as novel parameters used to tailor patient care through nanodiagnostics, individualized risk stratification, and therapeutic monitoring. Despite an increasing development of AFM biomedical research with patient cells, showing its unique capabilities in terms of resolution, speed, and accuracy, there is a notable need for applied AFM research in clinical settings. More translational research with AFM may provide new grounds for the valuable collaboration between biomedical researchers and healthcare professionals.
Keywords: Atomic force microscopy; Cancer; Cardiovascular diseases; Infectious diseases; Neurodegenerative diseases.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures




References
-
- Abramczyk H, Imiela A, Brozek-Pluska B, Kopec M (2019) Advances in Raman imaging combined with AFM and fluorescence microscopy are beneficial for oncology and cancer research. Nanomedicine 14(14):1873–1888. 10.2217/nnm-2018-0335 - PubMed
-
- Aguayo S, Marshall H, Pratten J, Bradshaw D, Brown JS, Porter SR, Spratt D, Bozec L (2017) Early adhesion of Candida albicans onto dental acrylic surfaces. J Dent Res 96(8):917–923. 10.1177/0022034517706354 - PubMed
-
- Ando T (2013) High-speed atomic force microscopy. J Electron Microsc 62(1):81–93. 10.1093/jmicro/dfs093 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

