Physical communication pathways in bacteria: an extra layer to quorum sensing
- PMID: 40376406
- PMCID: PMC12075086
- DOI: 10.1007/s12551-025-01290-1
Physical communication pathways in bacteria: an extra layer to quorum sensing
Abstract
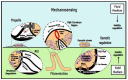
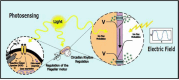
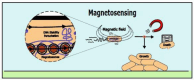
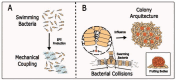
Bacterial communication is essential for survival, adaptation, and collective behavior. While chemical signaling, such as quorum sensing, has been extensively studied, physical cues play a significant role in bacterial interactions. This review explores the diverse range of physical stimuli, including mechanical forces, electromagnetic fields, temperature, acoustic vibrations, and light that bacteria may experience with their environment and within a community. By integrating these diverse communication pathways, bacteria can coordinate their activities and adapt to changing environmental conditions. Furthermore, we discuss how these physical stimuli modulate bacterial growth, lifestyle, motility, and biofilm formation. By understanding the underlying mechanisms, we can develop innovative strategies to combat bacterial infections and optimize industrial processes.
Keywords: Bacterial communication; Bacterial sensing; Bacterial signaling; Emerging properties; Environmental adaptation; Physical stimuli.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures




Similar articles
-
Synergy between c-di-GMP and Quorum-Sensing Signaling in Vibrio cholerae Biofilm Morphogenesis.J Bacteriol. 2022 Oct 18;204(10):e0024922. doi: 10.1128/jb.00249-22. Epub 2022 Sep 26. J Bacteriol. 2022. PMID: 36154360 Free PMC article.
-
Quorum Sensing: Not Just a Bridge Between Bacteria.Microbiologyopen. 2025 Mar;14(1):e70016. doi: 10.1002/mbo3.70016. Microbiologyopen. 2025. PMID: 40159675 Free PMC article. Review.
-
Communication within East Antarctic Soil Bacteria.Appl Environ Microbiol. 2019 Dec 13;86(1):e01968-19. doi: 10.1128/AEM.01968-19. Print 2019 Dec 13. Appl Environ Microbiol. 2019. PMID: 31628145 Free PMC article.
-
Bioorganic compounds in quorum sensing disruption: strategies, Mechanisms, and future prospects.Bioorg Chem. 2025 Mar;156:108192. doi: 10.1016/j.bioorg.2025.108192. Epub 2025 Jan 23. Bioorg Chem. 2025. PMID: 39874908 Review.
-
Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities.Int J Mol Sci. 2024 Nov 28;25(23):12808. doi: 10.3390/ijms252312808. Int J Mol Sci. 2024. PMID: 39684519 Free PMC article. Review.
References
-
- Ahmed I, Istivan T, Cosic I, Pirogova E (2013) Evaluation of the effects of extremely low frequency (ELF) pulsed electromagnetic fields (PEMF) on survival of the bacterium Staphylococcus aureus. EPJ Nonlinear Biomed Phys 1(1):5. 10.1140/epjnbp12
Publication types
LinkOut - more resources
Full Text Sources

