Skeletal Muscle Tissue Engineering: From Tissue Regeneration to Biorobotics
- PMID: 40376483
- PMCID: PMC12079140
- DOI: 10.34133/cbsystems.0279
Skeletal Muscle Tissue Engineering: From Tissue Regeneration to Biorobotics
Abstract
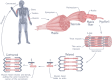
With its remarkable adaptability, energy efficiency, and mechanical compliance, skeletal muscle is a powerful source of inspiration for innovations in engineering and robotics. Originally driven by the clinical need to address large irreparable muscle defects, skeletal muscle tissue engineering (SMTE) has evolved into a versatile strategy reaching beyond medical applications into the field of biorobotics. This review highlights recent advancements in SMTE, including innovations in scaffold design, cell sourcing, usage of external physicochemical cues, and bioreactor technologies. Furthermore, this article explores the emerging synergies between SMTE and robotics, focusing on the use of robotic systems to enhance bioreactor performance and the development of biohybrid devices integrating engineered muscle tissue. These interdisciplinary approaches aim to improve functional recovery outcomes while inspiring novel biohybrid technologies at the intersection of engineering and regenerative medicine.
Copyright © 2025 Maira Z. Cordelle et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




Similar articles
-
Global Evolution of Skeletal Muscle Tissue Engineering: A Scientometric Research.Tissue Eng Part C Methods. 2021 Sep;27(9):497-511. doi: 10.1089/ten.TEC.2021.0156. Tissue Eng Part C Methods. 2021. PMID: 34445889
-
Stem Cells for Skeletal Muscle Tissue Engineering.Tissue Eng Part B Rev. 2018 Oct;24(5):373-391. doi: 10.1089/ten.TEB.2017.0451. Epub 2018 Apr 19. Tissue Eng Part B Rev. 2018. PMID: 29652595 Review.
-
Biomaterials-Based Technologies in Skeletal Muscle Tissue Engineering.Adv Healthc Mater. 2024 Jul;13(18):e2304196. doi: 10.1002/adhm.202304196. Epub 2024 May 20. Adv Healthc Mater. 2024. PMID: 38712598 Review.
-
Trends and Future Research in Skeletal Muscle Tissue Engineering in the Past Decade (2012-2022).Tissue Eng Part C Methods. 2024 Mar;30(3):130-141. doi: 10.1089/ten.TEC.2023.0216. Tissue Eng Part C Methods. 2024. PMID: 38265015
-
Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering.ACS Biomater Sci Eng. 2022 Feb 14;8(2):379-405. doi: 10.1021/acsbiomaterials.1c01145. Epub 2022 Jan 27. ACS Biomater Sci Eng. 2022. PMID: 35084836 Free PMC article. Review.
References
-
- Philips C, Terrie L, Thorrez L. Decellularized skeletal muscle: A versatile biomaterial in tissue engineering and regenerative medicine. Biomaterials. 2022;283: Article 121436. - PubMed
-
- Frontera WR, Ochala J. Skeletal muscle: A brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–195. - PubMed
-
- Duffy RM, Feinberg AW. Engineered skeletal muscle tissue for soft robotics: Fabrication strategies, current applications, and future challenges. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(2):178–195. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

