Frizzled receptors: gatekeepers of Wnt signaling in development and disease
- PMID: 40376615
- PMCID: PMC12078226
- DOI: 10.3389/fcell.2025.1599355
Frizzled receptors: gatekeepers of Wnt signaling in development and disease
Abstract
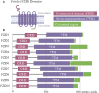
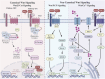
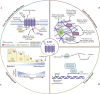
Frizzled (FZD) receptors are a subset of G-protein-coupled receptors (GPCRs), the largest class of human cell surface receptors and a major target of FDA-approved drugs. Activated by Wnt ligands, FZDs regulate key cellular processes such as proliferation, differentiation, and polarity, positioning them at the intersection of developmental biology and disease, including cancer. Despite their significance, FZD signaling remains incompletely understood, particularly in distinguishing receptor-specific roles across canonical and non-canonical Wnt pathways. Challenges include defining ligand-receptor specificity, elucidating signal transduction mechanisms, and understanding the influence of post translational modifications and the cellular context. Structural dynamics, receptor trafficking, and non-canonical signaling contributions also remain areas of active investigation. Recent advances in structural biology, transcriptomics, and functional genomics are beginning to address these gaps, while emerging therapeutic approaches-such as small-molecule modulators and antibodies-highlight the potential of FZDs as drug targets. This review synthesizes current insights into FZD receptor biology, examines ongoing controversies, and outlines promising directions for future research and therapeutic development.
Keywords: DVL; FZD; Wnt; cancer; frizzled receptor; signaling.
Copyright © 2025 Martinez-Marin, Stroman, Fulton and Pruitt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Frizzled receptors (FZDs) in Wnt signaling: potential therapeutic targets for human cancers.Acta Pharmacol Sin. 2024 Aug;45(8):1556-1570. doi: 10.1038/s41401-024-01270-3. Epub 2024 Apr 17. Acta Pharmacol Sin. 2024. PMID: 38632318 Free PMC article. Review.
-
Frizzleds act as dynamic pharmacological entities.Trends Pharmacol Sci. 2024 May;45(5):419-429. doi: 10.1016/j.tips.2024.03.003. Epub 2024 Apr 8. Trends Pharmacol Sci. 2024. PMID: 38594145 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Deciphering complexity of GPCR signaling and modulation: implications and perspectives for drug discovery.Clin Sci (Lond). 2025 May 20;139(10):463-77. doi: 10.1042/CS20245182. Clin Sci (Lond). 2025. PMID: 40400289 Free PMC article. Review.
-
Relationship between Pituitary Gland and Stem Cell in the Aspect of Hormone Production and Disease Prevention: A Narrative Review.Endocr Metab Immune Disord Drug Targets. 2025;25(7):509-526. doi: 10.2174/0118715303314551241031093717. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39812047 Review.
Cited by
-
Wnt signal pathways: new mechanistic approaches and clinical horizons in cancer therapy.Med Oncol. 2025 Jul 8;42(8):314. doi: 10.1007/s12032-025-02868-1. Med Oncol. 2025. PMID: 40629193 Review.
References
-
- Agajanian M. J., Walker M. P., Axtman A. D., Ruela-de-Sousa R. R., Serafin D. S., Rabinowitz A. D., et al. (2019). WNT activates the AAK1 kinase to promote clathrin-mediated endocytosis of LRP6 and establish a negative feedback loop. Cell Rep. 26 (1), 79–93.e8. 10.1016/j.celrep.2018.12.023 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

