Ixekizumab Retention Rate and Predictors of Treatment Persistence in Psoriatic Arthritis: Results of an Italian Multicenter Study
- PMID: 40376617
- PMCID: PMC12075152
- DOI: 10.1177/24755303251342503
Ixekizumab Retention Rate and Predictors of Treatment Persistence in Psoriatic Arthritis: Results of an Italian Multicenter Study
Abstract
IXE (Ixekizumab) is a monoclonal antibody targeting interleukin-17A (IL17A) which has demonstrated significant efficacy and safety in the management of psoriatic arthritis (PsA) in randomized controlled trials (RCTs). However, available data on long-term persistence of therapy are scarce.
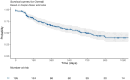
Methods: This multi-center study aimed to evaluate the drug retention rate (DRR) of IXE in a real-world setting and to identify key factors influencing treatment persistence. 195 patients with PsA treated with IXE between 2018 and 2024 were included. The primary outcome was DRR, calculated at 360, 720, and 1080 days after treatment initiation. Clinical and demographic factors were analyzed as potential predictors of IXE treatment permanency.
Results: IXE retention rates were 66% at 360 days, 49% at 720 days, and 39% at 1080 days. Low baseline disease activity was a strong predictor of higher retention (HR 0.24, 95% CI: 0.09-0.62, p = 0.003), while younger age was significantly associated with improved persistence (HR 0.98, 95% CI: 0.96-1.00, p = 0.045). Conversely, patients with both axial and peripheral joint involvement were more likely to discontinue therapy (HR 1.78, 95% CI: 1.04-3.06, p = 0.036), as were those receiving IXE as a second- or third-line therapy (HR 1.17, 95% CI: 1.02-1.33, p = 0.021).
Conclusions: This multicenter real-world study confirms the long-term retention rate of IXE in PsA. The findings highlight key factors influencing treatment persistence and provide valuable insights to optimize patient management. Further real-world research is needed to better understand the therapeutic performance of IXE in different patient populations.
Keywords: biologic treatment; comparative effectiveness; interleukin 17 inhibitor; ixekizumab; psoriasis; psoriatic arthritis.
© The Author(s) 2025.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ariani A has received honoraria as a speaker and an advisory board member for AbbVie, Abiogen, Amgen, Bristol-Myers Squibb, Boehringer, Bruno Farmaceutici, Stada EG, Janssen, Lilly, Novartis, Novo Nordisk, Sanofi, and Zentiva. Lumetti F has received honoraria as an advisory board member for Amgen. None of the other authors have any potential conflicts of interest to disclose in relation to this work.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
