Mechanically and Chemically Defined PEG Hydrogels Improve Reproducibility in Human Cardioid Development
- PMID: 40376871
- PMCID: PMC12184082
- DOI: 10.1002/adhm.202403997
Mechanically and Chemically Defined PEG Hydrogels Improve Reproducibility in Human Cardioid Development
Abstract
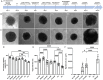
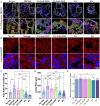
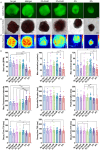
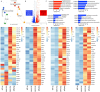
Cardioids are 3D self-organized heart organoids directly derived from induced pluripotent stem cells (hiPSCs) aggregates. The growth and culture of cardioids is either conducted in suspension culture or heavily relies on Matrigel encapsulation. Despite the significant advancements in cardioid technology, reproducibility remains a major challenge, limiting their widespread use in both basic research and translational applications. Here, for the first time, we employed synthetic, matrix metalloproteinase (MMP)-degradable polyethylene glycol (PEG)-based hydrogels to define the effect of mechanical and biochemical cues on cardioid development. Successful cardiac differentiation is demonstrated in all the hydrogel conditions, while cardioid cultured in optimized PEG hydrogel (3 wt.% PEG-2mM RGD) underwent similar morphological development and comparable tissue functions to those cultured in Matrigel. Matrix stiffness and cell adhesion motif play a critical role in cardioid development, nascent chamber formation, contractile physiology, and endothelial cell gene enrichment. More importantly, synthetic hydrogel improved the reproducibility in cardioid properties compared to traditional suspension culture and Matrigel encapsulation. Therefore, PEG-based hydrogel has the potential to be used as an alternative to Matrigel for human cardioid culture in a variety of clinical applications including cell therapy and tissue engineering.
Keywords: cardioids; human induced pluripotent stem cells; mechanobiology; organoids; synthetic hydrogel.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mensah G. A., Roth G. A., Fuster V., J. Am. Coll. Cardiol. 2019, 74, 2529. - PubMed
-
- Drakhlis L., Biswanath S., Farr C.‐M., Lupanow V., Teske J., Ritzenhoff K., Franke A., Manstein F., Bolesani E., Kempf H., Liebscher S., Schenke‐Layland K., Hegermann J., Nolte L., Meyer H., de la Roche J., Thiemann S., Wahl‐Schott C., Martin U., Zweigerdt R., Nat. Biotechnol. 2021, 39, 737. - PMC - PubMed
-
- Drakhlis L., Devadas S. B., Zweigerdt R., Nat. Protoc. 2021, 16, 5652. - PubMed
-
- Hofbauer P., Jahnel S. M., Papai N., Giesshammer M., Deyett A., Schmidt C., Penc M., Tavernini K., Grdseloff N., Meledeth C., Ginistrelli L. C., Ctortecka C., Salic S., Novatchkova M., Mendjan S., Cell 2021, 184, 3299. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

