Gossypin induces apoptosis and autophagy via the MAPK/JNK pathway in HT‑29 human colorectal cancer cells
- PMID: 40376978
- PMCID: PMC12101101
- DOI: 10.3892/ijmm.2025.5548
Gossypin induces apoptosis and autophagy via the MAPK/JNK pathway in HT‑29 human colorectal cancer cells
Abstract
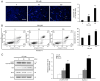
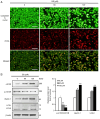
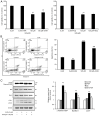
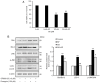
Gossypin, a flavone found in Hibiscus vitifolius, exhibits antioxidant, antidiabetic, anti‑inflammatory and anticancer effects. The present study investigated the potential of gossypin to induce apoptosis and autophagy in HT‑29 human colorectal cancer (CRC) cells, and assessed its association with the MAPK/JNK pathway. Cell viability assays, DAPI staining, flow cytometry, acridine orange staining, western blotting, hematoxylin and eosin staining, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and immunohistochemistry were performed. The results revealed an increased number of apoptotic bodies, higher apoptosis rates and enhanced autophagy in gossypin‑treated HT‑29 cells. To investigate autophagy during cell death, the effects of the early autophagy inhibitor 3‑methyladenine (3‑MA) and the late autophagy inhibitor hydroxychloroquine on cell viability and the expression of apoptosis‑related proteins were assessed. Significant increases in cell viability were observed following 3‑methyladenine pretreatment, as well as a decrease in the expression levels of Bcl‑2 and an increase in Bax. The analysis of MAPK pathway proteins following treatment with gossypin revealed that the levels of phosphorylated (p‑)JNK and p‑p38 were significantly increased in a concentration‑dependent manner. The JNK inhibitor SP600125 was used to confirm the role of the JNK pathway in gossypin‑induced apoptosis and autophagy. Moreover, gossypin reduced the volume of HT‑29 tumors in mice, and western blotting indicated the induction of apoptosis and autophagy in these tumors in vivo. Finally, TUNEL and immunohistochemistry experiments confirmed the induction of apoptosis and p‑JNK upregulation in these tumors in vivo. In conclusion, the present study suggested that gossypin may induce MAPK/JNK‑mediated apoptosis and autophagy in HT‑29 CRC cells, highlighting the potential of gossypin as an anticancer agent.
Keywords: HT‑29; MAPK/JNK pathway; apoptosis; autophagy; colorectal cancer; gossypin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Myricetin induces apoptosis through the MAPK pathway and regulates JNK‑mediated autophagy in SK‑BR‑3 cells.Int J Mol Med. 2022 Apr;49(4):54. doi: 10.3892/ijmm.2022.5110. Epub 2022 Mar 2. Int J Mol Med. 2022. PMID: 35234274 Free PMC article.
-
Novel quinazolinone MJ‑33 induces AKT/mTOR‑mediated autophagy‑associated apoptosis in 5FU‑resistant colorectal cancer cells.Oncol Rep. 2021 Feb;45(2):680-692. doi: 10.3892/or.2020.7882. Epub 2020 Dec 3. Oncol Rep. 2021. PMID: 33416156 Free PMC article.
-
Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells.Oncol Rep. 2019 Nov;42(5):1904-1914. doi: 10.3892/or.2019.7295. Epub 2019 Aug 28. Oncol Rep. 2019. PMID: 31485597 Free PMC article.
-
Gambogic acid induces apoptosis and inhibits colorectal tumor growth via mitochondrial pathways.World J Gastroenterol. 2015 May 28;21(20):6194-205. doi: 10.3748/wjg.v21.i20.6194. World J Gastroenterol. 2015. PMID: 26034354 Free PMC article.
-
RA-XII, a bicyclic hexapeptidic glucoside isolated from Rubia yunnanensis Diels, exerts antitumor activity by inhibiting protective autophagy and activating Akt-mTOR pathway in colorectal cancer cells.J Ethnopharmacol. 2021 Feb 10;266:113438. doi: 10.1016/j.jep.2020.113438. Epub 2020 Oct 2. J Ethnopharmacol. 2021. PMID: 33017635
References
-
- WHO Colorectal cancer. 2023. https://www.who.int/newsroom/fact-sheets/detail/colorectal-cancer .
-
- Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, Thorsen AJ, Weiser MR, Chang GJ, Lightner AL, et al. The American society of colon and rectal surgeons clinical practice guidelines for the management of colon cancer. Dis Colon Rectum. 2022;65:148–177. doi: 10.1097/DCR.0000000000002323. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
