Curcumenol inhibits malignant progression and promotes ferroptosis via the SLC7A11/NF‑κB/TGF‑β pathway in triple‑negative breast cancer
- PMID: 40377003
- PMCID: PMC12121984
- DOI: 10.3892/ijmm.2025.5552
Curcumenol inhibits malignant progression and promotes ferroptosis via the SLC7A11/NF‑κB/TGF‑β pathway in triple‑negative breast cancer
Abstract
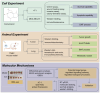
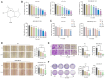
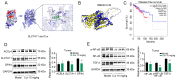
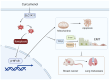
Triple‑negative breast cancer (TNBC) exhibits a high degree of malignancy and a propensity for metastasis, ultimately resulting in unfavorable patient outcomes. Curcuma phaeocaulis Valeton is a common herb used in traditional Chinese medicine to treat TNBC. Curcumenol (Cur) is a natural compound derived from C. phaeocaulis Valeton, the effects of which on breast cancer remain under‑reported. The present study elucidated that Cur could effectively inhibit the survival ability of TNBC cells and enhance their sensitivity to paclitaxel. Western blotting (WB) further revealed that Cur modulated apoptosis and epithelial‑mesenchymal transition (EMT) in TNBC. Findings from animal experiments further validated these observations. In the established TNBC mouse model, Cur was shown to exert an inhibitory effect on tumor growth, effectively attenuate EMT and substantially reduce the incidence of lung metastasis. Integrated analyses using RNA sequencing, WB and reverse transcription‑quantitative polymerase chain reaction demonstrated that Cur markedly downregulated the expression levels of solute carrier family 7 member 11 (SLC7A11), phosphorylated‑NF‑κB and TGF‑β. Molecular docking studies further validated that Cur can establish stable interactions with SLC7A11. In‑depth bioinformatics analysis revealed a positive association between high SLC7A11 expression and reduced disease‑free survival in patients with breast cancer. Additionally, in TNBC cells, Cur was revealed to reduce the mitochondrial membrane potential and promote the accumulation of lipid reactive oxygen species. Subsequent experimental investigations demonstrated that Cur can counteract the inhibitory influence of ferrostatin‑1 on ferroptosis. These findings strongly implied a potential underlying mechanism, suggesting that Cur may impede the malignant progression of TNBC via the modulation of ferroptosis. In conclusion, the findings of the present study underscore the marked efficacy of Cur in hampering the progression of TNBC by suppressing the SLC7A11/NF‑κB/TGF‑β signaling pathway.
Keywords: curcumenol; epithelial‑mesenchymal transition; ferroptosis; lung metastasis; triple‑negative breast cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The deubiquitinase OTUD5 stabilizes SLC7A11 to promote progression and reduce paclitaxel sensitivity in triple-negative breast cancer.Cancer Lett. 2024 Nov 1;604:217232. doi: 10.1016/j.canlet.2024.217232. Epub 2024 Sep 12. Cancer Lett. 2024. PMID: 39276913
-
Curcumin suppresses colorectal cancer by induction of ferroptosis via regulation of p53 and solute carrier family 7 member 11/glutathione/glutathione peroxidase 4 signaling axis.Phytother Res. 2024 Aug;38(8):3954-3972. doi: 10.1002/ptr.8258. Epub 2024 Jun 4. Phytother Res. 2024. PMID: 38837315
-
RSL3 induces ferroptosis by activating the NF-κB signalling pathway to enhance the chemosensitivity of triple-negative breast cancer cells to paclitaxel.Sci Rep. 2025 Jan 11;15(1):1654. doi: 10.1038/s41598-025-85774-w. Sci Rep. 2025. PMID: 39794456 Free PMC article.
-
Discovery of a natural small-molecule compound that suppresses tumor EMT, stemness and metastasis by inhibiting TGFβ/BMP signaling in triple-negative breast cancer.J Exp Clin Cancer Res. 2019 Mar 21;38(1):134. doi: 10.1186/s13046-019-1130-2. J Exp Clin Cancer Res. 2019. PMID: 30898152 Free PMC article.
-
Apatinib combined with paclitaxel suppresses synergistically TNBC progression through enhancing ferroptosis susceptibility regulated SLC7A11/GPX4/ACSL4 axis.Cell Signal. 2025 Jul;131:111760. doi: 10.1016/j.cellsig.2025.111760. Epub 2025 Mar 20. Cell Signal. 2025. PMID: 40120963
Cited by
-
Adipo-Modulation by Turmeric Bioactive Phenolic Components: From Curcuma Plant to Effects.Int J Mol Sci. 2025 Jul 17;26(14):6880. doi: 10.3390/ijms26146880. Int J Mol Sci. 2025. PMID: 40725127 Free PMC article. Review.
References
-
- Joaquin Garcia A, Rediti M, Venet D, Majjaj S, Kammler R, Munzone E, Gianni L, Thürlimann B, Laáng I, Colleoni M, et al. Differential benefit of metronomic chemotherapy among triple-negative breast cancer subtypes treated in the IBCSG trial 22-00. Clin Cancer Res. 2023;29:4908–4919. doi: 10.1158/1078-0432.CCR-23-1267. - DOI - PubMed
-
- Rusakiewicz S, Tyekucheva S, Tissot-Renaud S, Chaba K, Imbimbo M, Benedetti F, Kammler R, Hornfeld J, Munzone E, Gianni L, et al. Multiplexed high-throughput immune cell imaging in patients with high-risk triple negative early breast cancer: Analysis from the international breast cancer study group (IBCSG) trial 22-00. Eur J Cancer. 2024;200:113535. doi: 10.1016/j.ejca.2024.113535. - DOI - PubMed
-
- Hanna D, Merrick S, Ghose A, Devlin MJ, Yang DD, Phillips E, Okines A, Chopra N, Papadimatraki E, Ross K, et al. Real world study of sacituzumab govitecan in metastatic triple-negative breast cancer in the United Kingdom. Br J Cancer. 2024;130:1916–1920. doi: 10.1038/s41416-024-02685-9. - DOI - PMC - PubMed
-
- Dent R, André F, Gonçalves A, Martin M, Schmid P, Schütz F, Kümmel S, Swain SM, Bilici A, Loirat D, et al. IMpassion132 double-blind randomised phase III trial of chemotherapy with or without atezolizumab for early relapsing unresectable locally advanced or metastatic triple-negative breast cancer. Ann Oncol. 2024;35:630–642. doi: 10.1016/j.annonc.2024.04.001. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
