Reactive Oxygen Species: From Tumorigenesis to Therapeutic Strategies in Cancer
- PMID: 40377005
- PMCID: PMC12082284
- DOI: 10.1002/cam4.70947
Reactive Oxygen Species: From Tumorigenesis to Therapeutic Strategies in Cancer
Abstract
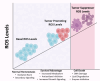
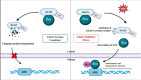
Background: Reactive oxygen species (ROS), a class of highly reactive molecules, are closely linked to the pathogenesis of various cancers. While ROS primarily originate from normal cellular processes, external stimuli can also contribute to their production. Cancer cells typically exhibit elevated ROS levels due to disrupted redox homeostasis, characterized by an imbalance between antioxidant and oxidant species. ROS play a dual role in cancer biology: at moderate levels, they facilitate tumor progression by regulating oncogenes and tumor suppressor genes, inducing mutations, promoting proliferation, extracellular matrix remodeling, invasion, immune modulation, and angiogenesis. However, excessive ROS levels can cause cellular damage and initiate apoptosis, necroptosis, or ferroptosis.
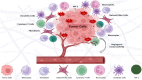
Methods: This review explores molecular targets involved in redox homeostasis dysregulation and examines the impact of ROS on the tumor microenvironment (TME). Literature from recent in vitro and in vivo studies was analyzed to assess how ROS modulation contributes to cancer development and therapy.
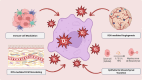
Results: Findings indicate that ROS influence cancer progression through various pathways and cellular mechanisms. Targeting ROS synthesis or enhancing ROS accumulation in tumor cells has shown promising anticancer effects. These therapeutic strategies exhibit significant potential to impair tumor growth while also interacting with elements of the TME.
Conclusion: The ROS serve as both promoters and suppressors of cancer depending on their intracellular concentration. Their complex role offers valuable opportunities for targeted cancer therapies. While challenges remain in precisely modulating ROS for therapeutic benefit, they hold promise as synergistic agents alongside conventional treatments, opening new avenues in cancer management.
Keywords: cancer; homeostatic dysregulation; reactive oxygen species; signal transducer; tumorigenesis.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Davies K. J., ed., “Oxidative Stress: The Paradox of Aerobic Life,” in Biochemical Society Symposia (Portland Press Limited, 1995). - PubMed
-
- Sies H., Belousov V. V., Chandel N. S., et al., “Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology,” Nature Reviews. Molecular Cell Biology 23, no. 7 (2022): 499–515. - PubMed
-
- Lambert A. J. and Brand M. D., “Reactive Oxygen Species Production by Mitochondria,” Mitochondrial DNA: Methods and Protocols 554 (2009): 165–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

