Nuclear Import Defects Drive Cell Cycle Dysregulation in Neurodegeneration
- PMID: 40377023
- PMCID: PMC12341789
- DOI: 10.1111/acel.70091
Nuclear Import Defects Drive Cell Cycle Dysregulation in Neurodegeneration
Abstract
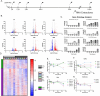
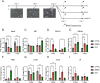
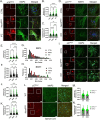
Neurodegenerative diseases (NDDs) and other age-related disorders have been classically defined by a set of key pathological hallmarks. Two of these hallmarks, cell cycle dysregulation (CCD) and nucleocytoplasmic transport (NCT) defects, have long been debated as being either causal or consequential in the pathology of accelerated aging. Specifically, aberrant cell cycle activation in post-mitotic neurons has been shown to trigger neuronal cell death pathways and cellular senescence. Additionally, NCT has been observed to be progressively dysregulated during aging and in neurodegeneration, where the increased subcellular redistribution of nuclear proteins, such as TAR DNA-Binding Protein-43 (TDP-43), to the cytoplasm is a primary driver of disease. However, the functional significance of NCT defects as either a causal mechanism or consequence of pathology, and how the redistribution of cell cycle machinery contributes to neurodegeneration, remains unclear. Here, we describe that pharmacological inhibition of importin-β nuclear import is capable of perturbing cell cycle machinery both in mitotic neuronal cell lines and post-mitotic primary neurons in vitro. Our NemfR86S mouse model of motor neuron disease, characterized by nuclear import defects, further recapitulates the hallmarks of CCD we observed in mitotic cell lines and in post-mitotic primary neurons in vitro, and in spinal motor neurons in vivo. The observed CCD is consistent with the transcriptional and phenotypical dysregulation commonly associated with neuronal cell death and senescence-like features in NDDs. Together, this evidence suggests that impairment of nuclear import pathways resulting in CCD may be a common driver of pathology in neurodegeneration.
Keywords: cell cycle; neurodegeneration; nuclear import.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
We permit the right to Wiley and Aging Cell to license and reproduce the above information. We required no permissions for any data or figures produced in this manuscript.
The authors declare no conflicts of interest.
Figures



Update of
-
Nuclear Import Defects Drive Cell Cycle Dysregulation in Neurodegeneration.bioRxiv [Preprint]. 2025 Jan 31:2025.01.28.635269. doi: 10.1101/2025.01.28.635269. bioRxiv. 2025. Update in: Aging Cell. 2025 Aug;24(8):e70091. doi: 10.1111/acel.70091. PMID: 39975276 Free PMC article. Updated. Preprint.
Similar articles
-
Nuclear Import Defects Drive Cell Cycle Dysregulation in Neurodegeneration.bioRxiv [Preprint]. 2025 Jan 31:2025.01.28.635269. doi: 10.1101/2025.01.28.635269. bioRxiv. 2025. Update in: Aging Cell. 2025 Aug;24(8):e70091. doi: 10.1111/acel.70091. PMID: 39975276 Free PMC article. Updated. Preprint.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Deficiency in KPNA4, but Not in KPNA3, Causes Attention Deficit/Hyperactivity Disorder like Symptoms in Mice.Genes (Basel). 2025 Jun 6;16(6):690. doi: 10.3390/genes16060690. Genes (Basel). 2025. PMID: 40565582 Free PMC article.
References
-
- Aranda‐Anzaldo, A. , and Dent M. A.. 2017. “Why Cortical Neurons Cannot Divide, and Why Do They Usually Die in the Attempt?” Journal of Neuroscience Research 95, no. 4: 921–929. - PubMed
-
- Baldassarre, G. , Belletti B., Nicoloso M. S., et al. 2005. “p27Kip1‐Stathmin Interaction Influences Sarcoma Cell Migration and Invasion.” Cancer Cell 7, no. 1: 51–63. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

