Chronic Vanadium Exposure Promotes Aggregation of Alpha-Synuclein, Tau and Amyloid Beta in Mouse Brain
- PMID: 40377064
- PMCID: PMC12082766
- DOI: 10.1111/jnc.70082
Chronic Vanadium Exposure Promotes Aggregation of Alpha-Synuclein, Tau and Amyloid Beta in Mouse Brain
Abstract
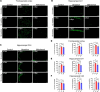
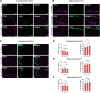
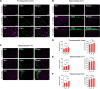
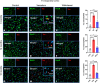
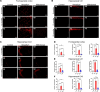
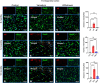
The interaction of toxic environmental metals and metalloids with brain proteins and polypeptides can result in pathological aggregations and formation of toxic oligomers, which are key features of many neurodegenerative diseases. Occupational and environmental exposure to vanadium is connected to a neurological syndrome that includes psychiatric symptoms, cognitive decline, and neurodegeneration. In this study, we hypothesized that prolonged vanadium exposure may be a potential risk factor for Alzheimer's and Parkinson's diseases. A total of 72 male BALB/c mice, each 4 weeks' old, were used. Vanadium-treated groups received intraperitoneal injections of 3 mg/kg body weight of vanadium three times a week for 6, 12, or 18 months. The control group received sterile water while the withdrawal group were given vanadium injection for 3 months, followed by withdrawal of the metal, but treatment with sterile water only, and were sacrificed at 3-, 9-, or 15-months post vanadium exposure. Sagittal sections of brain paraffin-embedded tissue were prepared and analyzed using immunofluorescence to study the immunoreactivity and cellular localization of α-synuclein (α-syn), amyloid-β (Aβ), and tau proteins. Our findings revealed pathological aggregation of these proteins in the frontoparietal cortices and the dorsal CA1 and CA3 regions. Double immunolabeling with glial cells and neurons showed neuronal degeneration, functional gliosis, and activation of astrocytes and microglia at sites of α-synuclein immunoreactivity. We observed increased immunoreactivity of phosphorylated nuclei tau both in the parietal cortices and corpus callosum white matter while we observed intraneuronal accumulation of Aβ deposits in the cortex and dorsal hippocampal regions in vanadium treated brains. These cellular changes and proteinopathies, although persisting, were significantly attenuated after vanadium withdrawal. Our findings show that prolonged vanadium exposure promotes abnormal accumulation of neurodegeneration-associated proteins (α-syn, Tau, and Aβ) in the brain, which is further exacerbated by aging in the context of extended exposure to the metal.
Keywords: aging; neurodegeneration; protein aggregation; proteinopathies; vanadium.
© 2025 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Afeseh Ngwa, H. , Kanthasamy A., Anantharam V., et al. 2009. “Vanadium Induces Dopaminergic Neurotoxicity via Protein Kinase Cdelta Dependent Oxidative Signaling Mechanisms: Relevance to Etiopathogenesis of Parkinson's Disease.” Toxicology and Applied Pharmacology 240, no. 2: 273–285. 10.1016/j.taap.2009.07.025. - DOI - PMC - PubMed
-
- Akash, M. S. H. , and Rehman K.. 2021. Environmental Contaminants and Neurological Disorders. Springer. - PubMed
-
- Alamri, S. H. , Haque S., Alghamdi B. S., et al. 2023. “Comprehensive Mapping of Mutations in TDP‐43 and α‐Synuclein That Affect Stability and Binding.” Journal of Biomolecular Structure and Dynamics 43: 1–13. - PubMed
-
- Antón‐Fernández, A. , Vallés‐Saiz L., Avila J., and Hernández F.. 2023. “Neuronal Nuclear Tau and Neurodegeneration.” Neuroscience 518: 178–184. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

