Histone Deacetylase 6 Controls Atrial Fibrosis and Remodeling in Postinfarction Mice Through the Modulation of Wnt3a/GSK-3β Signaling
- PMID: 40377174
- PMCID: PMC12083057
- DOI: 10.1096/fj.202500371R
Histone Deacetylase 6 Controls Atrial Fibrosis and Remodeling in Postinfarction Mice Through the Modulation of Wnt3a/GSK-3β Signaling
Abstract
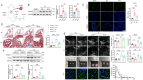
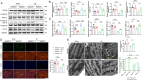
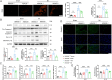
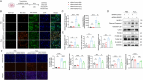
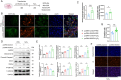
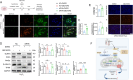
Myocardial infarction (MI)-induced hemodynamic disorder often causes atrial structural and electrophysiological remodeling. Given that histone deacetylase 6 (HDAC6) plays important roles in pathobiology, we investigated the molecular mechanism underlying MI-induced atrial remodeling in mice, with a special focus on HDAC6-mediated Wnt3a/GSK3β signaling activation. We observed an upregulation of HDAC6 expression in the left atria of mice at 2 weeks post-MI, accompanied by atrial enlargement, increased atrial fibrosis and inflammation, myocyte hypertrophy, impaired mitochondrial biogenesis, elevated levels of Wnt3a, GSK3β, and β-catenin protein, and reduced gap junction CX43 expression; these alterations were reversed by HDAC6 deletion. This atrialoprotective effect was mimicked by HDAC6 inhibition with the HDAC6 inhibitor tubastatin A (TubA). In HL1 mouse atrial myocytes, HDAC6 silencing (or overexpression) reduced (increased) the Wnt3a and p-GSK3β protein levels, providing evidence and a mechanistic explanation of HDAC6-mediated Wnt3a/GSK3β signaling activation in mitochondrial oxidative stress production and cell pyroptosis. After HDAC6 formed a complex with GSK3β and translocated into the mitochondria, GSK3β competitively bound with TFAM to mtDNA, thereby affecting mitochondrial function and ROS generation. The SGLT2 inhibitor dapagliflozin exhibited efficacy that was comparable to that of TubA by inhibiting HDAC6 signaling in mice. These results indicate an essential role of HDAC6 in atrial remodeling in response to post-MI stress, possibly via the modulation of Wnt3a/GSK3β-mediated mitochondrial oxidative stress production and pyroptosis and matrix protein production, and they suggest a novel therapeutic strategy for the prevention of post-MI-related atrial morphological and electrophysiological remodeling by regulating HDAC6 activity.
Keywords: atrial remodeling; fibrosis; mitochondria; myocardial infarction; oxidative stress.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Histone deacetylase 6 controls cardiac fibrosis and remodelling through the modulation of TGF-β1/Smad2/3 signalling in post-infarction mice.J Cell Mol Med. 2024 Sep;28(17):e70063. doi: 10.1111/jcmm.70063. J Cell Mol Med. 2024. PMID: 39232846 Free PMC article.
-
Genetic deletion or pharmacologic inhibition of histone deacetylase 6 protects the heart against ischaemia/reperfusion injury by limiting tumour necrosis factor alpha-induced mitochondrial injury in experimental diabetes.Cardiovasc Res. 2024 Oct 14;120(12):1456-1471. doi: 10.1093/cvr/cvae144. Cardiovasc Res. 2024. PMID: 39001869 Free PMC article.
-
Chronic HDAC6 Activation Induces Atrial Fibrillation Through Atrial Electrical and Structural Remodeling in Transgenic Mice.Int Heart J. 2021;62(3):616-626. doi: 10.1536/ihj.20-703. Int Heart J. 2021. PMID: 34054002
-
OCT4B-190 protects against ischemic stroke by modulating GSK-3β/HDAC6.Exp Neurol. 2019 Jun;316:52-62. doi: 10.1016/j.expneurol.2019.04.005. Epub 2019 Apr 11. Exp Neurol. 2019. PMID: 30981804
-
Inhibition of tartrate-resistant acid phosphatase 5 can prevent cardiac fibrosis after myocardial infarction.Mol Med. 2024 Jun 15;30(1):89. doi: 10.1186/s10020-024-00856-1. Mol Med. 2024. PMID: 38879488 Free PMC article.
Cited by
-
Increased Dipeptidyl Peptidase-4 Promotes Adipose Inflammation and Dysfunction in Mice Under Chronic Stress.FASEB J. 2025 Aug 15;39(15):e70893. doi: 10.1096/fj.202502147R. FASEB J. 2025. PMID: 40742315 Free PMC article.
References
-
- Schmitt J., Duray G., Gersh B. J., and Hohnloser S. H., “Atrial Fibrillation in Acute Myocardial Infarction: A Systematic Review of the Incidence, Clinical Features and Prognostic Implications,” European Heart Journal 30 (2009): 1038–1045. - PubMed
-
- Montero‐Cabezas J. M., Abou R., Chimed S., et al., “Effects of Atrial Ischemia on Left Atrial Remodeling in Patients With ST‐Segment Elevation Myocardial Infarction,” Journal of the American Society of Echocardiography 36 (2023): 163–171. - PubMed
-
- Tubeeckx M. R. L., De Keulenaer G. W., Heidbuchel H., and Segers V. F. M., “Pathophysiology and Clinical Relevance of Atrial Myopathy,” Basic Research in Cardiology 119 (2024): 215–242. - PubMed
-
- De Jong A. M., Maass A. H., Oberdorf‐Maass S. U., Van Veldhuisen D. J., Van Gilst W. H., and Van Gelder I. C., “Mechanisms of Atrial Structural Changes Caused by Stretch Occurring Before and During Early Atrial Fibrillation,” Cardiovascular Research 89 (2011): 754–765. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

