The antimicrobial peptide Cec4 has therapeutic potential against clinical carbapenem-resistant Klebsiella pneumoniae
- PMID: 40377314
- PMCID: PMC12210888
- DOI: 10.1128/spectrum.02738-24
The antimicrobial peptide Cec4 has therapeutic potential against clinical carbapenem-resistant Klebsiella pneumoniae
Abstract
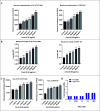
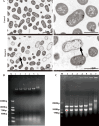
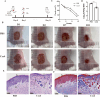
The rapid increase in carbapenem-resistant Klebsiella pneumoniae (CRKP) infections, along with the cross-resistance of CRKP to other antibiotics, has created an urgent need for novel therapeutic agents. Among the potential options for next-generation antibiotics, antimicrobial peptides (AMPs) show great promise. In this study, we aimed to elucidate the mechanisms underlying the antibacterial activity against CRKP of an antibacterial peptide named Cecropin-4 (Cec4), which we successfully identified previously. Our results demonstrate that Cec4 not only exhibits rapid antibacterial activity but also effectively inhibits and eradicates bacterial biofilm at a low concentration of 8 µg/mL. Additionally, when used in combination with traditional antibiotics, Cec4 enhances their antibacterial effect. Microscopy techniques, including transmission electron microscopy (TEM), confocal laser scanning microscopy, and scanning electron microscopy (SEM), found that Cec4 destroyed bacteria's cell membrane integrity and increased the membrane permeability (flow cytometry instrument technology further characterization of Cec4 against K. pneumoniae bacteria antibacterial effect). Furthermore, in vitro experiments demonstrated that Cec4 binds to bacterial DNA and RNA of CRKP. Moreover, in vivo studies using a mouse skin wound model confirmed the efficacy of Cec4, and transcriptomic analysis shed light on the molecular mechanisms underlying its antibacterial activity. Based on our findings, Cec4 appears to be a promising candidate for combating CRKP infections.IMPORTANCEThe rapid increase in carbapenem-resistant Klebsiella pneumoniae (CRKP) infections and the serious cross-resistance to multiple antibiotics make the development of new therapeutic drugs urgent. Antimicrobial peptides (AMPs) have attracted much attention as a potential option for the next generation of antibiotics. Previous studies have identified the antimicrobial peptide Cecropin-4 (Cec4), and this study further explored its antimicrobial mechanism against CRKP. Studies have found that Cec4 shows high antibacterial activity at low concentrations, can inhibit and eradicate bacterial biofilms, and can also enhance the efficacy of traditional antibiotics. Its mechanism of action, such as destroying cell membranes and binding nucleic acid, has been revealed by various techniques, and its effectiveness has been confirmed in vivo, providing a promising candidate drug for combating CRKP infection.
Keywords: CRKP; Klebsiella pneumoniae; antibacterial activity; antibacterial mechanism; antimicrobial peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Antibacterial efficacy of cefoperazone/sulbactam in combination with various antimicrobials against carbapenem-resistant Klebsiella pneumoniae.Acta Microbiol Immunol Hung. 2025 Apr 23;72(2):106-112. doi: 10.1556/030.2025.02577. Print 2025 Jun 20. Acta Microbiol Immunol Hung. 2025. PMID: 40266683
-
Molecular epidemiology and clinical characteristics of carbapenem-resistant Klebsiella pneumoniae bloodstream and pneumonia isolates.Microbiol Spectr. 2025 Aug 5;13(8):e0063125. doi: 10.1128/spectrum.00631-25. Epub 2025 Jul 9. Microbiol Spectr. 2025. PMID: 40631745 Free PMC article.
-
A comparative analysis of clinical outcomes in hematological patients afflicted with bacteremia attributable to carbapenem-resistant Klebsiella pneumoniae versus Escherichia coli.Front Cell Infect Microbiol. 2025 Jun 17;15:1600746. doi: 10.3389/fcimb.2025.1600746. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40599648 Free PMC article.
-
Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae.Ann Clin Microbiol Antimicrob. 2017 Mar 29;16(1):18. doi: 10.1186/s12941-017-0191-3. Ann Clin Microbiol Antimicrob. 2017. PMID: 28356109 Free PMC article.
-
Efficacy and safety of ceftazidime-avibactam compared to other antimicrobials for the treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae strains, a systematic review and meta-analysis.Microb Pathog. 2023 Jun;179:106090. doi: 10.1016/j.micpath.2023.106090. Epub 2023 Mar 31. Microb Pathog. 2023. PMID: 37004964
References
-
- Musicha P, Cornick JE, Bar-Zeev N, French N, Masesa C, Denis B, Kennedy N, Mallewa J, Gordon MA, Msefula CL, Heyderman RS, Everett DB, Feasey NA. 2017. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998-2016): a surveillance study. Lancet Infect Dis 17:1042–1052. doi: 10.1016/S1473-3099(17)30394-8 - DOI - PMC - PubMed
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group . 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 82002180, 81660347/Natural Science Foundation for Young Scientists of Shanxi Province
- ZK[2022] Key Program 039/Science and Technology Program of Guizhou Province
- 2022MD723770/China Postdoctoral Science Foundation
- 21NSFCP39/Guizhou Medical University
- gzwkj2023-567/Department of Health of Guizhou Province
LinkOut - more resources
Full Text Sources
Medical

