Light and polyphosphate kinase 2 cooperatively regulate the production of zero-valent sulfur in a deep-sea bacterium
- PMID: 40377319
- PMCID: PMC12172448
- DOI: 10.1128/msystems.00473-25
Light and polyphosphate kinase 2 cooperatively regulate the production of zero-valent sulfur in a deep-sea bacterium
Abstract
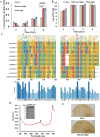
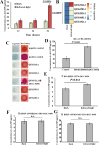
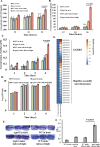
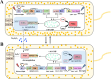
It is well established that different wavelengths of light exist in various deep-sea environments, and many deep-sea microorganisms have evolved specialized mechanisms for sensing and utilizing light energy. Our previous research found that blue light promotes zero-valent sulfur (ZVS) production in Erythrobacter flavus 21-3, a bacterium isolated from a deep-sea cold seep. Given that long-wavelength light is more prevalent in deep-sea environments, the present study investigates the mechanism by which E. flavus 21-3 senses infrared light (wavelength 940 nm) and regulates ZVS production. We found that the bacteriophytochrome BPHP-15570 is responsible for sensing infrared light, which induces autophosphorylation of BPHP-15570, activating the diguanylate cyclase DGC-0450 for c-di-GMP biosynthesis. Subsequently, the PilZ domain-containing protein mPilZ-1753 binds to c-di-GMP, triggering a well-established ZVS production pathway involving thiosulfate dehydrogenase (TsdA) and two homologs of thiosulfohydrolases (SoxB). Notably, polyphosphate kinase 2 (PPK2) is recruited to compete for GTP, the direct precursor of c-di-GMP biosynthesis. This competition downregulates ZVS production as well as other important metabolic processes. This negative regulatory pathway helps the bacterium avoid excessive ZVS accumulation, which could be toxic to bacterial growth. Overall, E. flavus 21-3 has evolved a sophisticated regulatory pathway to sense both blue and infrared light, triggering ZVS production. Our study provides a valuable model for understanding light utilization and its coupling with sulfur cycling in deep-sea environments.IMPORTANCEIt is widely believed that deep-sea ecosystems operate independently of light, relying primarily on chemical energy. However, the discovery of non-photosynthetic bacteria in various deep-sea environments that can sense and utilize light has challenged this assumption. In a recent study, we found that blue light significantly promotes the production of zero-valent sulfur (ZVS) in the deep-sea bacterium Erythrobacter flavus 21-3. Given that long-wavelength light is more prevalent in deep-sea environments, we investigated whether infrared light also plays a role in regulating ZVS production in E. flavus 21-3. Our results indicate that infrared light does promote ZVS formation in this bacterium. We identified PPK2 as a negative regulator, maintaining intracellular ZVS at safe levels to prevent toxicity due to excessive accumulation. Overall, our study offers a valuable model for exploring how light is utilized and its interaction with microbial sulfur cycling in the extreme conditions of the deep sea.
Keywords: c-di-GMP; deep-sea bacterium; infrared light; polyphosphate kinase 2; zero-valent sulfur.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Blue light promotes zero-valent sulfur production in a deep-sea bacterium.EMBO J. 2023 Jun 15;42(12):e112514. doi: 10.15252/embj.2022112514. Epub 2023 Mar 22. EMBO J. 2023. PMID: 36946144 Free PMC article.
-
Functional analysis of cyclic diguanylate-modulating proteins in Vibrio fischeri.mSystems. 2024 Nov 19;9(11):e0095624. doi: 10.1128/msystems.00956-24. Epub 2024 Oct 22. mSystems. 2024. PMID: 39436151 Free PMC article.
-
The cyclic di-GMP receptor YcgR links the second messenger with the putrescine quorum sensing system in modulation of Dickeya oryzae motility.mBio. 2025 Jul 9;16(7):e0101625. doi: 10.1128/mbio.01016-25. Epub 2025 May 30. mBio. 2025. PMID: 40444987 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Vitamin D: Production, Metabolism, and Mechanism of Action.2025 Jun 15. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. 2025 Jun 15. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. PMID: 25905172 Free Books & Documents. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

