Two-year outcomes of epicranial focal cortex stimulation in pharmacoresistant focal epilepsy
- PMID: 40377414
- PMCID: PMC12455469
- DOI: 10.1111/epi.18448
Two-year outcomes of epicranial focal cortex stimulation in pharmacoresistant focal epilepsy
Abstract
Objective: This study was undertaken to report on the long-term safety and efficacy of epicranial focal cortex stimulation (FCS) using the EASEE device as adjunctive neuromodulatory therapy in improving seizure control in adults with pharmacoresistant epilepsy originating from one predominant epileptogenic zone.
Methods: Prospective open-label follow-up of patients from the EASEE II and PIMIDES I clinical trials was done for a period of 2 years after the epicranial implantation of the EASEE electrode and stimulator device.
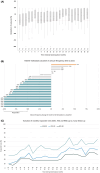
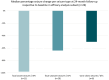
Results: Thirty-three patients underwent device implantation, and stimulation was activated in 32 patients. Of these, 26 patients continued stimulation up to 2-year follow-up and provided seizure diary data for efficacy analysis. The 50% responder rate at 2-year follow-up was 65.4% (95% confidence interval = 44.3-82.8), corresponding to a median seizure frequency reduction of 68%. Patients reported improved health-related quality of life. Tolerability was excellent, and there were no severe adverse events considered to be related to implantation or stimulation, nor were adverse effects on mood or cognition reported.
Significance: Results of the 2-year follow-up show that epicranial FCS is well tolerated by patients while providing improved seizure control in the long term. It thus offers a minimally invasive treatment option for patients with a predominant epileptic focus.
Keywords: epileptic focus; focal epilepsy; long‐term outcome; neuromodulation; neurostimulation.
© 2025 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
A.S.‐B. has received research support from Bial, Precisis, and UNEEG, and personal honoraria for lectures or advice from Angelini Pharma, Eisai, Jazz Pharmaceuticals, Precisis, UCB, and UNEEG. M.H. has received lecture fees or grants from UCB, Angelini Pharma, and UNEEG. A.M. has received consultancy fees from LivaNova and speaker fees from Angelini Pharma. E.K. has received personal honoraria for lectures or advice from UCB, UNEEG, Medtronic, Desitin, and Precisis and has participated in trials sponsored by Medtronic, UCB, Ergomed, and Precisis. V.C. receives a collaborative grant from Brainlab. He serves as an advisor for CereGate and CorTec. He has an ongoing investigator‐initiated trial with Boston Scientific. M.G. has received honoraria and educational grants from Precisis, LivaNova, Abbott, Medtronic, Boston Scientific, and Nevro. S.G. reports research funding from patient groups, Bundesministerium für Bildung und Forschung, DFG (SPP2177 Radiomics), UM Mainz, Abbott, Boston Scientific, Böhringer Foundation, Magventure, National MS Society, Precisis, and Innovationsfond GBA (01NVF22107, INSPIRE
Figures

References
-
- Schulze‐Bonhage A, Nitsche MA, Rotter S, Focke NK, Rao VR. Neurostimulation targeting the epileptic focus: current understanding and perspectives for treatment. Seizure. 2024;117:183–192. - PubMed
-
- Schulze‐Bonhage A. Intracranial and epicranial focus stimulation–concepts and approval status–English Version. Clin Epileptol. 2023;36:125–129.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

