Systemic anticancer therapy at the end of life: real-world insights from a tertiary oncology center in Israel
- PMID: 40377438
- PMCID: PMC12082831
- DOI: 10.1093/oncolo/oyaf066
Systemic anticancer therapy at the end of life: real-world insights from a tertiary oncology center in Israel
Abstract
Background: Aggressive end-of-life (EOL) care, such as systemic anticancer therapy (SACT) for advanced cancer patients, represents a potential indicator of low-quality care that may deviate from the primary palliative objective of treatment.
Methods: A retrospective study analyzed consecutive patients with advanced cancers treated at a tertiary oncology center in Israel from January 2019 to December 2022. Demographic and clinical data were examined, with a focus on intravenous (IV) oncologic treatment administration rates at 30 and 90 days before death.
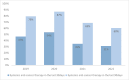
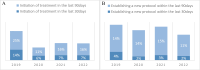
Results: The study included 1851 patients who received IV oncologic medications and died during 2019-2022. The median age at death was 69 years, with 51.3% (951) being men. Systemic anticancer therapy administration rates were 36% (666 patients) in the last 30 days and 67.6% (1252 patients) in the last 90 days prior to death. Chemotherapy was the most common EOL medication (58%). Higher EOL SACT rates were associated with younger age, better ECOG performance status, shorter disease duration, and specific tumor origins, particularly breast cancer. Conversely, gender, marital status, and ethnicity showed no significant correlation with EOL treatment use.
Discussion: Our data provide insight into current practice adopted by healthcare professionals regarding EOL treatment administration in Israel. A positive EOL experience is a significant goal in the oncology clinic, yet our findings demonstrate high rates of aggressive EOL care and may highlight the necessity for regulatory and educational changes within the healthcare system.
Keywords: aggressive care; end of life; good death; palliative care; systemic anticancer therapy.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
Authors have no financial conflicts of interest to disclose.
Figures
Similar articles
-
Systemic anticancer therapy near the end of life: an analysis of factors influencing treatment in advanced tumor disease.ESMO Open. 2024 Sep;9(9):103683. doi: 10.1016/j.esmoop.2024.103683. Epub 2024 Aug 29. ESMO Open. 2024. PMID: 39214050 Free PMC article.
-
Anticancer therapy at end-of-life: A retrospective cohort study.Acta Oncol. 2024 May 8;63:313-321. doi: 10.2340/1651-226X.2024.22139. Acta Oncol. 2024. PMID: 38716486 Free PMC article.
-
Palliative Radiotherapy Near the End of Life: An Analysis of Factors Influencing the Administration of Radiotherapy in Advanced Tumor Disease.JCO Glob Oncol. 2025 Apr;11:e2400500. doi: 10.1200/GO-24-00500. Epub 2025 Apr 18. JCO Glob Oncol. 2025. PMID: 40249890
-
Palliative Chemotherapy Near the End of Life in Oncology Patients.Am J Hosp Palliat Care. 2018 Sep;35(9):1215-1220. doi: 10.1177/1049909118763338. Epub 2018 Mar 12. Am J Hosp Palliat Care. 2018. PMID: 29529885
-
Systemic Anticancer Treatment Near the End of Life: a Narrative Literature Review.Curr Treat Options Oncol. 2023 Oct;24(10):1328-1350. doi: 10.1007/s11864-023-01115-x. Epub 2023 Jul 27. Curr Treat Options Oncol. 2023. PMID: 37501037 Free PMC article. Review.
References
-
- Lundberg FE, Andersson TML, Lambe M, et al.Trends in cancer survival in the Nordic countries 1990–2016: the NORDCAN survival studies. Acta Oncol. 2020;59:1266-1274. https://doi.org/10.1080/0284186x.2020.1822544 - DOI - PubMed
-
- Bright CJ, Lawton S, Benson S, et al.Data resource profile: the Systemic Anti-Cancer Therapy (SACT) dataset. Int J Epidemiol. 2020;49:15-15l. https://doi.org/10.1093/ije/dyz137 - DOI - PMC - PubMed
-
- Vanbutsele G, Pardon K, Van Belle S, et al.Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol. 2018;19:394-404. https://doi.org/10.1016/S1470-2045(18)30060-3 - DOI - PubMed
-
- Wadhwa D, Hausner D, Popovic G, et al.Systemic anti-cancer therapy use in palliative care outpatients with advanced cancer. J Palliat Care. 2021;36:78-86. https://doi.org/10.1177/0825859720975949 - DOI - PubMed
-
- Kerekes DM, Frey AE, Prsic EH, et al.Immunotherapy initiation at the end of life in patients with metastatic cancer in the US. JAMA Oncology. 2024;10:342-351. https://doi.org/10.1001/jamaoncol.2023.6025 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

