HBV-specific T-cell function is nonenhanced by tenofovir-induced decline in HBV viremia or HBsAg titer in chronic hepatitis B
- PMID: 40377525
- PMCID: PMC12088633
- DOI: 10.1097/HC9.0000000000000694
HBV-specific T-cell function is nonenhanced by tenofovir-induced decline in HBV viremia or HBsAg titer in chronic hepatitis B
Abstract
Background: Chronic hepatitis B is associated with virus-specific and global T-cell dysfunction. We hypothesized that therapeutic reduction in serum HBV DNA, ALT, and HBsAg would restore HBV-specific T-cell function and modify T-cell regulatory phenotype, with associated posttreatment ALT flare.
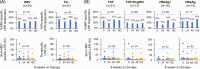
Methods: HBV-specific T-cell lymphoproliferative responses and global T-cell phenotype were prospectively examined at baseline, weeks 24, 48, 192, 216, and 240 in 34 adults with immune-active chronic hepatitis B treated with 192 weeks of tenofovir alone (n=21) or combined with pegylated interferon (PegIFN) in the first 24 weeks (n=13). HBV-specific T-cell IFNγ responses at weeks 0, 24, and 48 were examined by ELISpot assay ex vivo in 24 patients. Posttreatment flare was defined by serum ALT >5 times the upper limit of normal.
Results: Tenofovir therapy did not promote sustained induction of HBV-specific T-cell proliferative responses, regardless of PegIFN therapy or decreased serum HBsAg, HBV DNA, or ALT levels. Instead, HBV-specific T-cell IFNγ responses declined significantly by 48 weeks of therapy (p=0.008). Posttreatment ALT flare was associated with higher baseline %PD1+/CD8 (p=0.019), %PD1+/CD4 (p=0.039), and %CTLA4+/CD4 (p=0.003) T cells compared to non-flares, but without associated HBsAg loss or increased HBV-specific T-cell responsiveness.
Conclusion: HBV-specific T-cell function was not restored after 192 weeks of tenofovir therapy and did not correlate with HBsAg levels before, during, or after therapy. Baseline global T-cell regulatory phenotype was a predictor for ALT flare post-therapy without associated HBsAg decline. These findings support the need for more novel immune-modulatory approaches to enhance HBV-specific T-cell responsiveness.
Keywords: antiviral therapy; functional cure; hepatitis flare; immune pathogenesis; interferon.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The following disclosures are made without a conflict of interest: Daniel Traum (none); David K. Wong (none); Abdus S. Wahed (none); Daryl T. Lau (consulting for and grants from Abott and Gilead); Richard K. Sterling (institutional grant funding from Roche, Abbott, Gilead); Norah A. Terrault (Institutional grant support from GSK, Roche-Genentech, Gilead Sciences, and Target and previously served on DSMB for Moderna); Mandana Khalili (grants and consulting fees from Gilead Sciences Inc and grants from Intercept Pharmaceuticals); William M. Lee (grant support from Gilead, Novo Nordisk, Lipocine, Salix, Akero, and Madrigal, and consulting for SK, Genentech, Pfizer, Veristat, and RAPT); Timothy M. Block (on the Board of Hepion Pharma, a co-founder and equity holder in Harlingen Life Sciences); Anna S.F. Lo (institutional grant support from Target), currently serves as an advisor/consultant to Brii Biosciences, Chroma, Grifols, Moderna, Novo Nordisk (DSMB), Pfizer, Precision Sciences, Roche, Target (unpaid), Virion, and Zenasbio); Kyong-Mi Chang (member of Data Monitoring Committee for Virion Therapeutics, previously served on the advisory board for GSK and Arbutus).
Figures





References
-
- Buster EHCJ, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135:459–467. - PubMed
MeSH terms
Substances
Grants and funding
- UL1 TR001111/TR/NCATS NIH HHS/United States
- U01 DK082943/DK/NIDDK NIH HHS/United States
- UL1 TR000058/TR/NCATS NIH HHS/United States
- U01 DK082867/DK/NIDDK NIH HHS/United States
- U01 DK082874/DK/NIDDK NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- U01 DK082919/DK/NIDDK NIH HHS/United States
- U01 DK082927/DK/NIDDK NIH HHS/United States
- U01 DK082872/DK/NIDDK NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- M01 RR000040/RR/NCRR NIH HHS/United States
- R01 AI047519/AI/NIAID NIH HHS/United States
- U01 DK082923/DK/NIDDK NIH HHS/United States
- U01 DK082871/DK/NIDDK NIH HHS/United States
- U01 DK082944/DK/NIDDK NIH HHS/United States
- U01 DK082864/DK/NIDDK NIH HHS/United States
- U01 DK082843/DK/NIDDK NIH HHS/United States
- U01 DK082863/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 DK082866/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

