Drug-induced second tumors: a disproportionality analysis of the FAERS database
- PMID: 40377769
- PMCID: PMC12084198
- DOI: 10.1007/s12672-025-02502-6
Drug-induced second tumors: a disproportionality analysis of the FAERS database
Abstract
Background: Drug-induced second tumors (DIST) refer to new primary cancers that develop during or after the treatment of an initial cancer due to the long-term effects of medications. As a severe long-term adverse event, DIST has gained widespread attention globally in recent years. With the increasing prevalence of cancer treatments and the prolonged survival of patients, drug-induced second tumors have become more prominent and pose a significant public health challenge. However, most existing studies have focused on individual drugs or small patient cohorts, lacking large-scale, real-world data evaluations. Particularly, the potential second-tumor risk of new drugs remains underexplored.
Objective: This study aims to systematically assess the adverse event signals between drugs and second tumors using the U.S. FDA Adverse Event Reporting System (FAERS) database, employing disproportionality analysis (DPA) methods. It particularly focuses on uncovering drugs that have not clearly labeled second-tumor risks.
Methods: Data from the FDA Adverse Event Reporting System (FAERS), covering reports from its inception to the third quarter of 2024, was retrieved. After data standardization, four disproportionality methods were used: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS). These methods assessed the correlation between azacitidine and adverse drug events (ADEs). Additionally, the Weibull Shape Parameter (WSP) was used to analyze the characteristic patterns of time-to-onset curves. Newly discovered signals were verified against FDA drug labels to confirm their novelty. The Weibull analysis was conducted to examine the temporal aspects of adverse event occurrences.
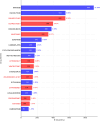
Results: Since 2004, drug-induced tumor events have been increasing annually, with a total of 7597 drug-related tumor adverse events recorded. A total of 250 drugs were identified as having potential risk signals. High-incidence populations were primarily aged between 65 and 85 years, with a higher proportion of individuals with a body weight ≥ 90 kg. The most frequent occurrence was observed in patients with Chronic Myeloid Leukemia (13.36%). Among the top 5 drugs with the highest number of reported drug-induced second tumor adverse events, IMATINIB (906 reports), RUXOLITINIB (554 reports), PALBOCICLIB (552 reports), OCTREOTIDE (399 reports), and DOXORUBICIN (380 reports) were identified. Among these, PALBOCICLIB, OCTREOTIDE, and DOXORUBICIN are drugs for which the risk of drug-induced second tumors is not explicitly mentioned in their labels. A total of 76 drugs were identified through four disproportionality algorithms (ROR, PRR, MGPS, BCPNN), with a minimum time to drug-induced tumor occurrence of 5 years, exhibiting an early failure-type curve.
Conclusion: This study, based on large-scale real-world data, reveals the potential associations between drugs and second tumors, especially highlighting the risks of some new drugs. The findings provide valuable insights for drug safety monitoring and have significant public health implications. By uncovering previously unrecognized potential risks, this research lays the groundwork for further advancements in pharmacovigilance.
Keywords: FAERS; Pharmacovigilance; Proportional imbalance analysis; Real world research; Second tumor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval and consent were not required as this study was based on publicly available data. Competing interests: The authors declare no competing interests.
Figures




References
-
- Katsimigas A, Pedersen LB, Haunstrup T, et al. Excellent clinical outcomes in deeply mutated IGHV chronic lymphocytic leukemia upon fludarabine and cyclophosphamide plus rituximab. Blood. 2024;144:1860. 10.1182/blood-2024-203046. - DOI
-
- Corazzelli G, Cuccaro A, Morelli E, et al. Long-term efficacy and safety of dose-dense and dose-intense ABVD without consolidation radiotherapy in patients with advanced hodgkin lymphoma: a 15-year follow-up of the ABVD(DD-DI) phase ii study. Br J Haematol. 2024;205:1383–8. 10.1111/bjh.19646. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
