Rapid Onset of Pain Relief with Crisugabalin in Patients with Diabetic Peripheral Neuropathic Pain: Findings from a Multicenter, Randomized, Double-Blind, Controlled Study
- PMID: 40377855
- PMCID: PMC12279680
- DOI: 10.1007/s40122-025-00745-3
Rapid Onset of Pain Relief with Crisugabalin in Patients with Diabetic Peripheral Neuropathic Pain: Findings from a Multicenter, Randomized, Double-Blind, Controlled Study
Abstract
Introduction: This study aims to evaluate the efficacy and safety of Crisugabalin in patients with diabetic peripheral neuropathic pain (DPNP), with a focus on its rapid onset of action.
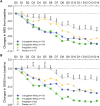
Methods: All the analyses in this study were based on data from a phase 2/3 adaptive randomized clinical trial that enrolled 596 patients. Participants were categorized into four treatment groups according to the intervention received: Crisugabalin 40 mg/day, Crisugabalin 80 mg/day, placebo, and Pregabalin 300 mg/day. The primary endpoint was the change in the average daily pain score (ADPS) over a 13-week treatment period. Secondary endpoints included changes in the Numeric Rating Scale (NRS) and the daily sleep interference score (DSIS) during the first two weeks of treatment.
Results: Both Crisugabalin treatment groups (40 mg/day and 80 mg/day) demonstrated statistically significant reductions in ADPS compared to the placebo group starting from week 1 and continuing through week 13 (P < 0.05). Significant differences in pain relief for the Pregabalin group were observed only from week 6. Improvements in NRS and DSIS scores were also noted in both Crisugabalin groups, with statistically significant enhancements evident as early as day 2 of administration. Safety assessments indicated that Crisugabalin was well-tolerated, with a low incidence of serious adverse events and no significant increase in dropout rates among participants.
Conclusion: The findings suggest that Crisugabalin offers effective pain relief with an acceptable safety profile, highlighting its rapid onset in patients with DPNP.
Clinical trial registration: Clinical trial registration number derived from our parent project, we have retained the original registration identifier: NCT04647773.
Keywords: Crisugabalin; Diabetic peripheral neuropathic pain (DPNP); Pain relief; Pregabalin; Rapid onset.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Fangqiong Li, Qin Huang, Yaming Li, and Zhixin Peng are full-time employees of Haisco Pharmaceutical Group Co., Ltd., the sponsor of this study. All other co-authors (Tianrong Pan, Jianhua Ma, Yukun Li, Kailiang Wang, Chengxia Jiang, Yawei Zhang, Jie Liu, Ruiqin Du, Wei Zhang, Fang Bian, Fang Zhang, Lijun Wang, Shuguang Pang, Tao Ning, Bangqiong Wang, Ya Li, Xiaohong Wu, Keqin Zhang, Xulei Tang, Honglin Hu, Xin Sun, Ping Li, Zhifeng Cheng, Jia Sun, Jing Yang, Yanjun Wang, Jialin Gao, Hong Mao, and Xiaohui Guo) declare no conflicts of interest relevant to this manuscript. Ethical Approval: The study was approved by the ethics committees or institutional review boards of the leading unit (the First Hospital of Peking University, Beijing, China) and other subcenters. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Participants provided their consent by signing a written informed consent form.
Figures

Similar articles
-
Antiepileptic drugs for chronic non-cancer pain in children and adolescents.Cochrane Database Syst Rev. 2017 Aug 5;8(8):CD012536. doi: 10.1002/14651858.CD012536.pub2. Cochrane Database Syst Rev. 2017. PMID: 28779491 Free PMC article.
-
Topical clonidine for neuropathic pain in adults.Cochrane Database Syst Rev. 2022 May 19;5(5):CD010967. doi: 10.1002/14651858.CD010967.pub3. Cochrane Database Syst Rev. 2022. PMID: 35587172 Free PMC article.
-
Methadone for neuropathic pain in adults.Cochrane Database Syst Rev. 2017 May 17;5(5):CD012499. doi: 10.1002/14651858.CD012499.pub2. Cochrane Database Syst Rev. 2017. PMID: 28514508 Free PMC article.
-
Tramadol for neuropathic pain in adults.Cochrane Database Syst Rev. 2017 Jun 15;6(6):CD003726. doi: 10.1002/14651858.CD003726.pub4. Cochrane Database Syst Rev. 2017. PMID: 28616956 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
Cited by
-
Therapeutic Potential of Calcium Channel Blockers in Neuropsychiatric, Endocrine and Pain Disorders.Cells. 2025 Jul 20;14(14):1114. doi: 10.3390/cells14141114. Cells. 2025. PMID: 40710367 Free PMC article. Review.
References
-
- Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. - PubMed
-
- Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17:400–20. - PubMed
-
- Lee CC, Perkins BA, Kayaniyil S, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the promise cohort. Diabetes Care. 2015;38(5):793–800. - PubMed
-
- Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH. Eurodiab prospective complications study group;. vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–50. - PubMed
-
- Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, Vinik AI, Boulton AJ. Toronto expert panel on diabetic neuropathy;. painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011;27:629–38. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

