Long-Term Transfusion Independence with Luspatercept Versus Epoetin Alfa in Erythropoiesis-Stimulating Agent-Naive, Lower-Risk Myelodysplastic Syndromes in the COMMANDS Trial
- PMID: 40377899
- PMCID: PMC12182481
- DOI: 10.1007/s12325-025-03208-5
Long-Term Transfusion Independence with Luspatercept Versus Epoetin Alfa in Erythropoiesis-Stimulating Agent-Naive, Lower-Risk Myelodysplastic Syndromes in the COMMANDS Trial
Abstract
Introduction: The efficacy of erythropoiesis-stimulating agents (ESAs) for transfusion-dependent (TD) anemia in lower-risk myelodysplastic syndromes (LR-MDS) is limited. Luspatercept achieved significantly greater rates of red blood cell (RBC) transfusion independence (TI) versus epoetin alfa (an ESA) in the phase 3 COMMANDS trial. This analysis assessed long-term RBC-TI, cumulative response, and safety with luspatercept in COMMANDS.
Methods: Eligible patients aged ≥ 18 years, with ESA-naive, RBC TD LR-MDS were randomized 1:1 to receive luspatercept (1.0 mg/kg, titration to 1.75 mg/kg permitted) or epoetin alfa (450 IU/kg, titration to 1050 IU/kg). Disease assessment was carried out at week 24 (day 169) and every 24 weeks thereafter. Treatment continued until disease progression, lack of clinical benefit, unacceptable toxicity, or consent withdrawal.
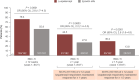
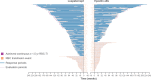
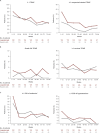
Results: At data cutoff (September 22, 2023; median follow-up: luspatercept 21.4 months, epoetin alfa 20.3 months), a greater proportion of patients treated with luspatercept (n = 182) versus epoetin alfa (n = 181) achieved a longest single RBC-TI period ≥ 1 year (44.5% vs. 27.6%; P = 0.0003) and ≥ 1.5 years (30.2% vs. 13.8%; P < 0.0001). Higher rates of RBC-TI ≥ 1.5 years with luspatercept over epoetin alfa were consistent across all prespecified subgroups, including patients with ring sideroblast-negative status and low baseline serum erythropoietin. Longer cumulative RBC-TI response [sum of all durations of RBC-TI for ≥ 12 weeks; week 1 to end of treatment (95% CI)] was observed with luspatercept [154.7 weeks (118.4-NR)] versus epoetin alfa [91.1 weeks (73.1-123.9)]. Rates of treatment-emergent adverse events, including asthenia and hypertension, generally decreased over time in both arms. Progression rates to high-risk MDS and acute myeloid leukemia were similarly low (< 5%) in both treatment arms.
Conclusions: These data demonstrated sustained, durable clinical benefit across subgroups and support luspatercept as the treatment of choice for anemia in patients with LR-MDS who are TD and ESA-naive.
Trial registration number: NCT03682536.
Keywords: Anemia; Epoetin alfa; Erythroid-stimulating agents; Luspatercept; Myelodysplastic syndromes; Transfusion-independence.
Plain language summary
Myelodysplastic syndromes (MDS) are a group of blood disorders, where the bone marrow fails to make enough healthy blood cells. MDS is also considered a blood cancer. Patients who have lower-risk MDS (LR-MDS) have a lower chance of progressing to more serious conditions, like high-risk MDS or acute myeloid leukemia. Most patients with LR-MDS have anemia (low red blood cells [RBC]) and need frequent RBC transfusions. Erythropoiesis-stimulating agents (ESAs), like epoetin alfa, are commonly used to treat anemia. However, ESAs may not be as effective in all patients and the effects may not last as long. The COMMANDS trial looked at patients with LR-MDS and compared 2 treatments, luspatercept and epoetin alfa. The aim was to see which treatment could help patients avoid transfusions for a longer time, or be “transfusion-free.” The results showed that there were more patients on luspatercept who were transfusion-free, compared with epoetin alfa, at both the 1-year and the 1.5-year timepoints. These results were similar across different patient subgroups, including patients with specific genetic mutations. Patients treated with luspatercept and epoetin alfa had similar rates of side effects, which decreased over time. Additionally, the risk of progression to more serious conditions was low and similar between the two groups. In conclusion, luspatercept helped patients with LR-MDS avoid transfusions for over 1.5 years. This supports the use of luspatercept as a first-choice treatment in patients with LR-MDS.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: Valeria Santini has been a member of the board of directors or an advisory board for AbbVie, Bristol Myers Squibb/Celgene, CTI, Geron, Jazz, Keros, Novartis, Servier, and Syros. Amer M. Zeidan has consulted for AbbVie, Agios, Akeso Pharma, ALX Oncology, Amgen, Astellas, BeiGene, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chiesi, Daiichi Sankyo, Epizyme, Faron, Genentech, Geron, Gilead, GlycoMimetics, Hikma, Janssen, Karyopharm, Kura Oncology, Kyowa Kirin, LAVA Therapeutics, Mendus, Notable Labs, Novartis, Orum, Otsuka, Pfizer, Regeneron, Rigel, Schrödinger, Servier, Sumitomo, Syndax, Syros, Taiho, Takeda, Treadwell, Vincerx, and Zentalis; has received research funding from AbbVie, Amgen, Astex, Bristol Myers Squibb, Celgene, Geron, Kura, Novartis, Otsuka, Shattuck Labs, Syros, and Takeda; and has received honoraria from AbbVie, Agios, Akeso Pharma, ALX Oncology, Amgen, Astellas, BeiGene, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chiesi, Daiichi Sankyo, Epizyme, Faron, Genentech, Geron, Gilead, GlycoMimetics, Hikma, Janssen, Karyopharm, Kura Oncology, Kyowa Kirin, LAVA Therapeutics, Mendus, Notable Labs, Novartis, Orum, Otsuka, Pfizer, Regeneron, Rigel, Schrödinger, Servier, Sumitomo, Syndax, Syros, Taiho, Takeda, Treadwell, Vincerx, and Zentalis. Rami S. Komrokji has participated in advisory boards for Bristol Myers Squibb, Daiichi Sankyo, Jazz, PharmaEssentia, Rigel, Servier, Sobi, and Sumitomo; has received research grants from Bristol Myers Squibb; has consulted for Geron and Genentech; and has participated in speakers’ bureaux for PharmaEssentia, Rigel, Servier, and Sobi. Veronika Pozharskaya is an employee of Bristol Myers Squibb and holds stock in Bristol Myers Squibb and Merck. Shelonitda Rose has received travel support from Bristol Myers Squibb and holds stock in Bristol Myers Squibb/Celgene. Yinzhi Lai, Sameer Kalsekar, Barkha Aggarwal, and Karen Keeperman are employees of and hold stock in Bristol Myers Squibb. Dimana Miteva is an employee of Bristol Myers Squibb. David Valcárcel has consulted for Amgen, Astellas, Bristol Myers Squibb/Celgene, Janssen, Jazz, Merck Sharpe & Dohme, Novartis, Pfizer, Sanofi, Sobi, and Takeda; has received honoraria from Amgen, Astellas, Bristol Myers Squibb/Celgene, Janssen, Jazz, Merck Sharpe & Dohme, Novartis, Pfizer, Sanofi, Sobi, and Takeda; and has received meeting and travel support from AbbVie, Bristol Myers Squibb/Celgene, Jazz, Novartis, and Sobi. Pierre Fenaux has received research funding (as the GFM chairperson) from AbbVie, Astex, Bristol Myers Squibb, Novartis, and Servier; and has received honoraria from AbbVie and Bristol Myers Squibb. Jake Shortt has received research funding from Astex/Taiho; has consulted for Astellas, Bristol Myers Squibb, Novartis, Otsuka, and Pfizer; has received honoraria from Novartis and Mundipharma; and has participated on the ELEMENT trial steering committee for Bristol Myers Squibb (paid to institution). Matteo Giovanni Della Porta has received honoraria from and participated on a data safety monitoring or advisory board for Bristol Myers Squibb. Uwe Platzbecker has received grant support (paid to GWT-TUD) from Amgen and Janssen; has received lecture fees and grant support (paid to the University of Leipzig) from Amgen; has received grant support (paid to the University of Dresden) from Merck and Novartis; has consulted for AbbVie, Bristol Myers Squibb, Curis, Geron, and Novartis; has received honoraria from Bristol Myers Squibb; has received travel support from Bristol Myers Squibb; and is a member of the board of directors or an advisory board for the MDS Foundation. Guillermo Garcia-Manero has nothing to disclose. Ethical Approval: The trial was performed in accordance with the Declaration of Helsinki and International Counsel on Harmonisation guidelines and received review board approval from each participating site’s institutional review board/ethics committee. All patients provided written informed consent documentation prior to trial entry.
Figures

References
-
- Braga Lemos M, Rodrigues SR, Schroeder T, Kulasekararaj AG, Matos JE, Tang D. Association between red blood cell transfusion dependence and burden in patients with myelodysplastic syndromes: a systematic literature review and meta-analysis. Eur J Haematol. 2021;107:3–23. - PubMed
-
- Hiwase DK, Singhal D, Strupp C, et al. Dynamic assessment of RBC-transfusion dependency improves the prognostic value of the revised-IPSS in MDS patients. Am J Hematol. 2017;92:508–14. - PubMed
-
- Santini V, Schemenau J, Levis A, et al. Can the revised IPSS predict response to erythropoietic-stimulating agents in patients with classical IPSS low or intermediate-1 MDS? Blood. 2013;122:2286–8. - PubMed
-
- Park S, Hamel JF, Toma A, et al. Outcome of lower-risk patients with myelodysplastic syndromes without 5q deletion after failure of erythropoiesis-stimulating agents. J Clin Oncol. 2017;35:1591–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

