Influence of Choline-Based Ionogel on Transdermal Delivery of Vancomycin Hydrochloride
- PMID: 40377918
- PMCID: PMC12135064
- DOI: 10.1021/acs.molpharmaceut.5c00255
Influence of Choline-Based Ionogel on Transdermal Delivery of Vancomycin Hydrochloride
Abstract
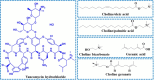
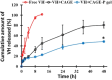
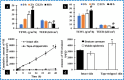
Ionic liquids (ILs) have attracted considerable interest as new drug delivery solvents because of their superior transdermal absorption of large molecular weight drugs across the biological barrier and their capacity to solubilize hydrophobic compounds. It is difficult to administer hydrophilic peptide treatments with large molecular weights through the skin. Vancomycin hydrochloride (VH) is a glycopeptide antibiotic that cures bacterial infections. With a molecular weight of 1449 Da and a high water solubility of 50 mg/mL, VH is an impressive compound. This study aimed to quantify the amount of VH transdermal penetration by analyzing the influence of choline-based ILs. Choline geranate was used as an IL in this investigation, whereas oleic acid (OA) (unsaturated) and palmitic acid (PA) (saturated) were chosen as the two types of fatty acids. The molar ratio of choline bicarbonate (CB) to either OA (CO) or PA (CP) was 2:1. In an additional series of trials, choline geranate (CAGE) ILs were prepared by mixing CB and geranic acid in a 1:2 molar ratio. For NMR spectroscopy, powder X-ray diffraction, differential scanning calorimetry, and Fourier transform infrared investigations, these formulations were characterized. Zeta potential indicated that all of the formulations had negative charges. The decreased irritation potential of CAGE, as shown by conductivity studies, makes it appropriate for skin application. To determine which formulation enhancers worked best, ex vivo skin permeation studies were carried out on both intact and tape-stripped skin. After 48 h, skin transport investigations showed that neat VH did not penetrate the excised porcine skin. Nevertheless, the CO and CP-based formulations greatly improved the skin penetration (6729 ± 437 μg/cm2) and retention (3892 ± 215 μg/g) of VH across the tape-stripped skin, whereas CAGE exhibited the most significant improvement (p < 0.05). Ionogel (CAGE-P) was finally fabricated by combining CAGE with 22.7% w/v Pluronic F-127 and 45.0% w/v PEG-400. The physical and rheological properties of VH-loaded CAGE-P gel were examined. The amount of VH permeated across the CAGE-P gel cotreated intact skin was 369 ± 41 μg/cm2, but that penetrated tape-stripped skin was 7543 ± 585 μg/cm2. The skin's barrier property underwent notable modifications (p < 0.05) following incubation with CAGE and CAGE-P gel formulations, as seen in the biophysical investigations conducted at various time intervals. Taken together, CAGE-Pluronic F-127 ionogel is promising and efficient as a topical formulation for the administration of VH in a localized and systemic manner.
Keywords: choline-geranate; dermal delivery; ionogel; oleic acid; palmitic acid; pluronic F-127; vancomycin hydrochloride.
Figures







Similar articles
-
Transdermal delivery of vancomycin hydrochloride: Influence of chemical and physical permeation enhancers.Int J Pharm. 2021 Jun 1;602:120663. doi: 10.1016/j.ijpharm.2021.120663. Epub 2021 Apr 30. Int J Pharm. 2021. PMID: 33933644
-
Ionic Liquid-Mediated Transdermal Delivery of Organogel Containing Cyclosporine A for the Effective Treatment of Psoriasis.ACS Omega. 2024 Sep 25;9(40):41565-41582. doi: 10.1021/acsomega.4c05346. eCollection 2024 Oct 8. ACS Omega. 2024. PMID: 39398161 Free PMC article.
-
Choline- versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: cytotoxicity, solubility, and skin permeation studies.Drug Dev Ind Pharm. 2017 Nov;43(11):1858-1865. doi: 10.1080/03639045.2017.1349788. Epub 2017 Jul 12. Drug Dev Ind Pharm. 2017. PMID: 28665154
-
Choline geranate (CAGE): A multifaceted ionic liquid for drug delivery.J Control Release. 2024 Dec;376:593-600. doi: 10.1016/j.jconrel.2024.10.034. Epub 2024 Oct 25. J Control Release. 2024. PMID: 39427776 Review.
-
Recent Developments in Ionic Liquid-Assisted Topical and Transdermal Drug Delivery.Pharm Res. 2022 Oct;39(10):2335-2351. doi: 10.1007/s11095-022-03322-x. Epub 2022 Jul 1. Pharm Res. 2022. PMID: 35773446 Review.
References
-
- Wu, K. ; Yeoh, T. ; Hsieh, Y.-L. ; Osborne, D. W. . Quality Assessment of API in Semisolid Topical Drug Products, 2019, pp 109–154.10.1007/978-3-030-17355-5_4. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

