Phase II Study Evaluating the Efficacy of Niraparib and Dostarlimab (TSR-042) in Patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma
- PMID: 40377969
- PMCID: PMC12146980
- DOI: 10.1158/2767-9764.CRC-25-0192
Phase II Study Evaluating the Efficacy of Niraparib and Dostarlimab (TSR-042) in Patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma
Abstract
Purpose: Recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) portends a poor prognosis. DNA pathway repair mutations in HNSCC are associated with higher tumor mutational burden rates and immune checkpoint inhibitor response. PARP inhibitors (PARPi) induce ssDNA breaks and are efficacious in cancers with DNA repair defects. Thus, we designed a single-arm, open-label, phase II clinical trial to evaluate the combination of niraparib and dostarlimab in patients with R/M HNSCC.
Patients and methods: Patients with R/M HNSCC were treated with niraparib and dostarlimab until disease progression or unacceptable toxicity. The primary endpoint was the overall response rate and clinical benefit assessed by RECIST version 1.1. Using Simon's two-step minimax design, 14 patients were planned to enroll in the first stage with a goal of overall clinical benefit of 50%.
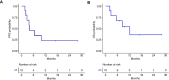
Results: Ten patients were enrolled. The majority were White males with a median age of 62.5. One patient had a PD-L1 combined positive score >20, a high tumor mutational burden, a BRCA1 rearrangement, and an ATRX splice site mutation. Nine patients previously failed anti-PD-1/PD-L1 therapy. The best overall response rate was 10%, with a 20% clinical benefit (1 partial response, 1 stable disease). The trial was terminated early for futility as the goal clinical benefit could not be reached. At a median follow-up of 10.13 months, the median progression-free survival was 3.8 months, and the median overall survival was 10.1 months. The most common grade 3 or higher treatment-related adverse events were thrombocytopenia and hypertension.
Conclusions: The combination of niraparib and dostarlimab did not achieve the primary endpoint of clinical benefit, but activity may be improved with biomarker-driven treatment and selected patients.
Significance: Patients with R/M HNSCC that progress on PD-1 inhibitors have poor prognoses. PARPis cause ssDNA breaks that accumulate in cells with mutations in DNA damage repair pathways, leading to synthetic lethality. However, PARPi also inhibits glycogen synthase kinase-3β activity, leading to upregulated PD-L1, which is abrogated by PD-1 inhibitors. In this study, we combine niraparib (PARPi) with dostarlimab (anti-PD-L1) to evaluate clinical benefit in patients with R/M HNSCC.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
O. Zamulko reports grants from GSK during the conduct of the study as well as other from Pfizer outside the submitted work. V. Karivedu reports a patent number 20240148725 pending. D. El-Gamal reports grants from AbbVie, the Nebraska Department of Health & Human Services Cancer and Smoking Disease Research Program, the Great Plains IDeA-CTR Network, and the American Cancer Society (ACS-IRG) and nonfinancial support from Opna Bio LLC outside the submitted work. T.M. Wise-Draper reports grants from GSK during the conduct of the study as well as grants and personal fees from Merck & Co; grants from Bristol Myers Squibb, Janssen, and AstraZeneca/MedImmune; and personal fees from EMD Serono, Adaptimmune, Replimune, Need Inc, High Enroll, and Caris Life Sciences outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- SEER Explorer . An interactive website for SEER cancer statistics. Surveillance Research Program. National Cancer Institute. [cited 2024 Apr 17]. Available from:https://seer.cancer.gov/statistics-network/explorer/.
-
- Thompson-Harvey A, Yetukuri M, Hansen AR, Simpson MC, Adjei Boakye E, Varvares MA, et al. . Rising incidence of late-stage head and neck cancer in the United States. Cancer 2020;126:1090–101. - PubMed
-
- Zheng F, Zhang Y, Chen S, Weng X, Rao Y, Fang H. Mechanism and current progress of poly ADP-ribose polymerase (PARP) inhibitors in the treatment of ovarian cancer. Biomed Pharmacother 2020;123:109661. - PubMed
-
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. . Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006;66:8109–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous