From blueprint to biobank: Leveraging expert recommendations for implementing change (ERIC) to pediatric cancer biobanking in Pakistan
- PMID: 40378096
- PMCID: PMC12083815
- DOI: 10.1371/journal.pone.0321316
From blueprint to biobank: Leveraging expert recommendations for implementing change (ERIC) to pediatric cancer biobanking in Pakistan
Abstract
Background: In low- and middle-income countries, limited infrastructure and resources hinder biobank establishment, affecting specimen diversity. Addressing this gap is crucial for equitable health outcomes, as current databases are skewed towards Northern-European populations. In Pakistan, pediatric cancer biobanks are non-existent. Indus Hospital & Health Network (IHHN) in Karachi, with its large pediatric cancer unit, aims to establish a biobank to address region-specific pediatric cancer research needs. This manuscript describes the biobank implementation process using implementation science frameworks.
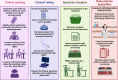
Methods: The pediatric cancer biobank at IHHN collects FFPE specimens for solid tumors, and isolated mononuclear cells from peripheral blood and bone marrow of suspected acute leukemia. Implementation planning workgroups included clinicians, EMR, IT, management, senior leadership, IRB, and external support from UNC and St. Jude Children's Cancer Hospital. The selection of applicable ERIC (Expert Recommendations for Implementing Change) strategies through stakeholder workgroups considered scope, budget, and feasibility, and context. Standard protocols from ISBER and BCNet guided alignment with best practices. IHHN's past experiences and tacit knowledge gained through rapid, successful implementation also facilitated strategy selection. The EPIS framework (exploration, preparation, implementation, sustainment) was used to map and organize the selected intervention strategies.
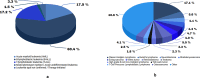
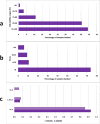
Results: Biobank implementation at IHHN, organized by EPIS stages, has been described through a set of 41 implementation strategies. Of these, 34 were selected out of 73 originally published ERIC strategies, while 7 were added based on contextually based workgroup consensus. 599 acute leukemia and 1137 solid tumor specimens have been banked since inception of the biobank operations 2 years earlier. The implementation activities and challenges described include infrastructure, swift specimen collection, prior to treatment, and informed consent. The ancillary processes including training and quality control have also been described and related data presented.
Conclusion: The implementation of Pakistan's first acute leukemia biobank using ERIC and EPIS frameworks offers a structured approach beneficial for settings with limited biobanking experience. This intervention aligns with recognized implementation science frameworks, while addressing aspects pertinent in low- and middle-income countries.
Copyright: © 2025 Aijaz et al.. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Plummer JT, George SHL. Challenges and opportunities in building a global representative single-cell and spatial atlas in cancer. Cancer Discov. 2023;13(9):1969–72. doi: 10.1158/2159-8290.CD-23-0810 - DOI - PubMed
-
- Annual Cancer Registry Report – 2017, of the Shaukat Khanum Memorial Cancer Hospital & Research Center, Pakistan. [cited 2024 Jun 2]. Available from: https://shaukatkhanum.org.pk/wp-content/uploads/2018/08/acrr-2017.pdf.
-
- Indus Hospital & Health Network. [cited 2024 Jun 2]. Available from: https://indushealthnetwork.org/
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

