The probability of reducing hospitalization rates for bronchiolitis with epinephrine and dexamethasone: A Bayesian analysis
- PMID: 40378135
- PMCID: PMC12083810
- DOI: 10.1371/journal.pone.0318853
The probability of reducing hospitalization rates for bronchiolitis with epinephrine and dexamethasone: A Bayesian analysis
Abstract
Background: Bronchiolitis exerts a high burden on children, their families and the healthcare system. The Canadian Bronchiolitis Epinephrine Steroid Trial (CanBEST) assessed whether administering epinephrine alone, dexamethasone alone, or in combination (EpiDex) could reduce bronchiolitis-related hospitalizations among children less than 12 months of age compared to placebo. CanBEST demonstrated a statistically significant reduction in 7-day hospitalization risk with EpiDex in an unadjusted analysis but not after adjustment.
Objective: To explore the probability that EpiDex results in a reduction in hospitalizations using Bayesian methods.
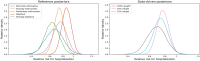
Study design: Using prior distributions that represent varying levels of preexisting enthusiasm or skepticism, i.e., how confident or doubtful one is that EpiDex may reduce hospitalizations, and information about the treatment effect before data were collected, the posterior distribution of the relative risk of hospitalization compared to placebo was determined. The probability that the treatment effect is less than 1, 0.9, 0.8 and 0.6, indicating increasing reductions in hospitalization risk, are computed alongside 95% credible intervals.
Results: Combining a minimally informative prior distribution with the data from CanBEST provides comparable results to the original analysis. Unless strongly skeptical views about the effectiveness of EpiDex were considered, the 95% credible interval for the treatment effect lies below 1, indicating a reduction in hospitalizations. There is a 90% probability that EpiDex results in a clinically meaningful reduction in hospitalization of 10% even when incorporating skeptical views, with a 67% probability when considering strongly skeptical views.
Conclusion: A Bayesian analysis demonstrates a high chance that EpiDex reduces hospitalization rates for bronchiolitis, although strongly skeptical individuals may require additional evidence to change practice.
Trial registration: Clinical Trial registry name, registration number: Current Controlled Trials number, ISRCTN56745572.
Copyright: © 2025 Dong et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Amy C. Plint receives in-kind support from Amphastar for a clinical trial in bronchiolitis. The other authors have no relevant conflicts to disclose.
Figures

References
-
- Shay D. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282(15):1440. - PubMed
-
- Langley JM, LeBlanc JC, Smith B, Wang EEL. Increasing incidence of hospitalization for bronchiolitis among Canadian children, 1980–2000. J Infect Dis. 2003;188(11):1764–7. - PubMed
-
- Njoo H, Pelletier L, Spika L. Infectious disease. Respiratory disease in Canada. Ottawa: Canadian Institute for Health Information, Canadian Lung Association, Health Canada, Statistics Canad; 2001. p. 65–84.
-
- Craig E, Jackson C, Han D, Grimwood K, NZCYES Steering Committee. Monitoring the health of New Zealand children and young people: Indicator handbook. Auckland, New Zealand: Paediatric Society of New Zealand and New Zealand Child and Youth Epidemiology Service. 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

