Nucleosomes represent a crucial target for the intra-S phase checkpoint in response to replication stress
- PMID: 40378213
- PMCID: PMC12083529
- DOI: 10.1126/sciadv.adr3673
Nucleosomes represent a crucial target for the intra-S phase checkpoint in response to replication stress
Abstract
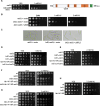
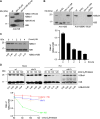
The intra-S phase checkpoint is essential for stability of stalled DNA replication forks. However, the mechanisms underlying checkpoint regulation remain poorly understood. This study identifies a critical checkpoint target-the ubiquitin E3 ligase Brl2, revealing a new dimension of checkpoint regulation. Upon replication fork stalling, Brl2 undergoes phosphorylation at five serine residues by Cds1Chk2 kinase, resulting in the loss of its ligase activity and a marked reduction in H2BK119ub1 levels. In the brl2-5D (the five serine residues are replaced with aspartic acid) and htb-K119R mutants, chromatin becomes highly compacted. Furthermore, the rates of stalled replication fork collapse, and dsDNA breaks are significantly reduced in brl2-5D cds1Chk2∆ cells compared to cds1Chk2∆ cells. Thus, this study demonstrates that nucleosomes are targeted by the intra-S phase checkpoint and highlights the checkpoint's critical role in configuring compact chromatin structures at replication fork stalling sites. These findings may explain why ATR and Chk1 are essential for cell proliferation and embryonic development, while ATM is not.
Figures





References
-
- Kornberg R. D., Lorch Y., Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98, 285–294 (1999). - PubMed
-
- Cardoso-Moreira M., Halbert J., Valloton D., Velten B., Chen C., Shao Y., Liechti A., Ascenção K., Rummel C., Ovchinnikova S., Mazin P. V., Xenarios I., Harshman K., Mort M., Cooper D. N., Sandi C., Soares M. J., Ferreira P. G., Afonso S., Carneiro M., Turner J. M. A., VandeBerg J. L., Fallahshahroudi A., Jensen P., Behr R., Lisgo S., Lindsay S., Khaitovich P., Huber W., Baker J., Anders S., Zhang Y. E., Kaessmann H., Gene expression across mammalian organ development. Nature 571, 505–509 (2019). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

