Ppp1r3b is a metabolic switch that shifts hepatic energy storage from lipid to glycogen
- PMID: 40378221
- PMCID: PMC12083521
- DOI: 10.1126/sciadv.ado3440
Ppp1r3b is a metabolic switch that shifts hepatic energy storage from lipid to glycogen
Abstract
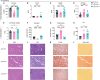
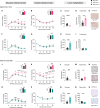
The PPP1R3B gene, encoding PPP1R3B protein, is critical for liver glycogen synthesis and maintaining blood glucose levels. Genetic variants affecting PPP1R3B expression are associated with several metabolic traits and liver disease, but the precise mechanisms are not fully understood. We studied the effects of both Ppp1r3b overexpression and deletion in mice and cell models and found that both changes in Ppp1r3b expression result in dysregulated metabolism and liver damage, with overexpression increasing liver glycogen stores, while deletion resulted in higher liver lipid accumulation. These patterns were confirmed in humans where variants increasing PPP1R3B expression had lower liver fat and decreased plasma lipids, whereas putative loss-of-function variants were associated with increased liver fat and elevated plasma lipids. These findings support that PPP1R3B is a crucial regulator of hepatic metabolism beyond glycogen synthesis and that genetic variants affecting PPP1R3B expression levels influence if hepatic energy is stored as glycogen or triglycerides.
Figures



References
-
- Younossi Z. M., Stepanova M., Ong J., Trimble G., AlQahtani S., Younossi I., Ahmed A., Racila A., Henry L., Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin. Gastroenterol. Hepatol. 19, 580–589.e5 (2021). - PubMed
-
- Manning A. K., Hivert M.-F., Scott R. A., Grimsby J. L., Bouatia-Naji N., Chen H., Rybin D., Liu C.-T., Bielak L. F., Prokopenko I., Amin N., Barnes D., Cadby G., Hottenga J.-J., Ingelsson E., Jackson A. U., Johnson T., Kanoni S., Ladenvall C., Lagou V., Lahti J., Lecoeur C., Liu Y., Martinez-Larrad M. T., Montasser M. E., Navarro P., Perry J. R. B., Rasmussen-Torvik L. J., Salo P., Sattar N., Shungin D., Strawbridge R. J., Tanaka T., van Duijn C. M., An P., de Andrade M., Andrews J. S., Aspelund T., Atalay M., Aulchenko Y., Balkau B., Bandinelli S., Beckmann J. S., Beilby J. P., Bellis C., Bergman R. N., Blangero J., Boban M., Boehnke M., Boerwinkle E., Bonnycastle L. L., Boomsma D. I., Borecki I. B., Böttcher Y., Bouchard C., Brunner E., Budimir D., Campbell H., Carlson O., Chines P. S., Clarke R., Collins F. S., Corbatón-Anchuelo A., Couper D., de Faire U., Dedoussis G. V., Deloukas P., Dimitriou M., Egan J. M., Eiriksdottir G., Erdos M. R., Eriksson J. G., Eury E., Ferrucci L., Ford I., Forouhi N. G., Fox C. S., Franzosi M. G., Franks P. W., Frayling T. M., Froguel P., Galan P., de Geus E., Gigante B., Glazer N. L., Goel A., Groop L., Gudnason V., Hallmans G., Hamsten A., Hansson O., Harris T. B., Hayward C., Heath S., Hercberg S., Hicks A. A., Hingorani A., Hofman A., Hui J., Hung J., Jarvelin M.-R., Jhun M. A., Johnson P. C. D., Wouter Jukema J., Jula A., Kao W. H., Kaprio J., Kardia S. L. R., Keinanen-Kiukaanniemi S., Kivimaki M., Kolcic I., Kovacs P., Kumari M., Kuusisto J., Kyvik K. O., Laakso M., Lakka T., Lannfelt L., Lathrop G. M., Launer L. J., Leander K., Li G., Lind L., Lindstrom J., Lobbens S., Loos R. J. F., Luan J., Lyssenko V., Mägi R., Magnusson P. K. E., Marmot M., Meneton P., Mohlke K. L., Mooser V., Morken M. A., Miljkovic I., Narisu N., O’Connell J., Ong K. K., Oostra B. A., Palmer L. J., Palotie A., Pankow J. S., Peden J. F., Pedersen N. L., Pehlic M., Peltonen L., Penninx B., Pericic M., Perola M., Perusse L., Peyser P. A., Polasek O., Pramstaller P. P., Province M. A., Räikkönen K., Rauramaa R., Rehnberg E., Rice K., Rotter J. I., Rudan I., Ruokonen A., Saaristo T., Sabater-Lleal M., Salomaa V., Savage D. B., Saxena R., Schwarz P., Seedorf U., Sennblad B., Serrano-Rios M., Shuldiner A. R., Sijbrands E. J. G., Siscovick D. S., Smit J. H., Small K. S., Smith N. L., Smith A. V., Stančáková A., Stirrups K., Stumvoll M., Sun Y. V., Swift A. J., Tönjes A., Tuomilehto J., Trompet S., Uitterlinden A. G., Uusitupa M., Vikström M., Vitart V., Vohl M.-C., Voight B. F., Vollenweider P., Waeber G., Waterworth D. M., Watkins H., Wheeler E., Widen E., Wild S. H., Willems S. M., Willemsen G., Wilson J. F., Witteman J. C. M., Wright A. F., Yaghootkar H., Zelenika D., Zemunik T., Zgaga L., DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, The Multiple Tissue Human Expression Resource (MUTHER) Consortium, Wareham N. J., McCarthy M. I., Barroso I., Watanabe R. M., Florez J. C., Dupuis J., Meigs J. B., Langenberg C., A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 44, 659–669 (2012). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

