Astrocytes-derived LCN2 triggers EV-A71-induced muscle soreness via accumulating lactate
- PMID: 40378229
- PMCID: PMC12083544
- DOI: 10.1126/sciadv.adt9837
Astrocytes-derived LCN2 triggers EV-A71-induced muscle soreness via accumulating lactate
Abstract
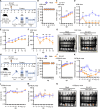
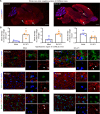
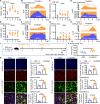
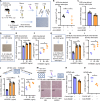
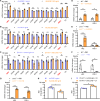
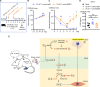
Viral muscle soreness (VMS) is a common feature during acute viral infections, including those caused by enteroviruses, and it substantially diminishes patients' quality of life. At present, we aim to establish the "brain-muscle" axis to explore the underlying mechanisms of VMS. We initially observed that diminished pain threshold occurred in enterovirus A71 (EV-A71)-infected C57BL/6J and AG6 mice. Subsequently, RNA sequencing data showed that lipocalin 2 (LCN2) was up-regulated during multiple viral infections, including EV-A71, Japanese encephalitis virus, vesicular stomatitis virus, and West Nile virus, which all caused VMS. As expected, Lcn2-deficient C57BL/6 J (Lcn2-/-) mice exhibited greater pain tolerance, as shown by stronger grip force and stable motor function after EV-A71 infection. Mechanistically, EV-A71-induced high-mobility group 1 (HMGB1) stimulated astrocyte-derived LCN2 secreted into the circulatory system, which enhanced glycolysis and induced lactate buildup in muscle through increasing pyruvate dehydrogenase kinase 1 (PDK1) expression and decreasing pyruvate dehydrogenase (PDH) activity. Together, HMGB1/LCN2/PDK1/lactate pathway in the brain-muscle axis promoted VMS development.
Figures




Similar articles
-
Astrocyte-secreted lipocalin-2 elicits bioenergetic failure-induced neuronal death that is causally related to high fatality in a mouse model of hepatic encephalopathy.Neurochem Int. 2024 Sep;178:105800. doi: 10.1016/j.neuint.2024.105800. Epub 2024 Jul 2. Neurochem Int. 2024. PMID: 38964718
-
Satellite glia as a critical component of diabetic neuropathy: Role of lipocalin-2 and pyruvate dehydrogenase kinase-2 axis in the dorsal root ganglion.Glia. 2021 Apr;69(4):971-996. doi: 10.1002/glia.23942. Epub 2020 Nov 29. Glia. 2021. PMID: 33251681
-
Metabolic Connection of Inflammatory Pain: Pivotal Role of a Pyruvate Dehydrogenase Kinase-Pyruvate Dehydrogenase-Lactic Acid Axis.J Neurosci. 2015 Oct 21;35(42):14353-69. doi: 10.1523/JNEUROSCI.1910-15.2015. J Neurosci. 2015. PMID: 26490872 Free PMC article.
-
Recent Advances in Enterovirus A71 Infection and Antiviral Agents.Lab Invest. 2024 Feb;104(2):100298. doi: 10.1016/j.labinv.2023.100298. Epub 2023 Nov 25. Lab Invest. 2024. PMID: 38008182 Review.
-
Lipocalin-2 as a therapeutic target for brain injury: An astrocentric perspective.Prog Neurobiol. 2016 Sep;144:158-72. doi: 10.1016/j.pneurobio.2016.08.001. Epub 2016 Aug 4. Prog Neurobiol. 2016. PMID: 27498195 Review.
Cited by
-
The Role of Lactylation in Virus-Host Interactions.Int J Mol Sci. 2025 Jul 10;26(14):6613. doi: 10.3390/ijms26146613. Int J Mol Sci. 2025. PMID: 40724863 Free PMC article. Review.
References
-
- Ślebioda Z., Dorocka-Bobkowska B., Hand, foot and mouth disease as an emerging public health problem: Case report of familial child-to-adult transmission. Dent. Med. Probl. 55, 99–104 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

