Early outcomes with a flexible ECAP based closed loop using multiplexed spinal cord stimulation waveforms-single-arm study with in-clinic randomized crossover testing
- PMID: 40378232
- PMCID: PMC12585107
- DOI: 10.1093/pm/pnaf058
Early outcomes with a flexible ECAP based closed loop using multiplexed spinal cord stimulation waveforms-single-arm study with in-clinic randomized crossover testing
Abstract
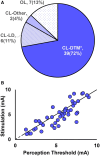
Background: Spinal cord stimulation (SCS) systems that deliver fixed amplitudes expose target tissue to varying electrical fields due to the changing lead-to-cord distance accompanying postural shifts and other body movements. Inconsistent stimulation results in periods of overstimulation or under-stimulation perceived by patients as discomfort or potentially inadequate pain relief. CL-SCS may be applied to provide a comfortable level of perception down to imperceptible stimulation, commonly preferred in higher frequency and multiplexed programming. Here we report outcomes from a study evaluating a closed-loop (CL) SCS system that uses spinal evoked compound action potentials to adjust stimulation.
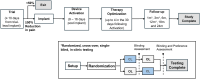
Methods: This ongoing study combines the evaluation of pain-related outcomes (for ≤24 months) with in-clinic randomized, crossover testing of CL performance.
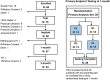
Results: Sixty subjects have been implanted with the CL-SCS system, and 54 subjects have completed the 3-month visit. Sixty percent preferred below-perception waveforms for therapy during at-home use. The study successfully met its primary endpoint with 89.3% of subjects in the Primary Analysis Set (n = 28) reporting reduction in overstimulation with CL-SCS relative to OL-SCS at 1 month (P < .001; binomial exact test); at 3 months, 86% of subjects with low-back/leg pain (n = 51); and all 3 with upper limb pain reported ≥50% reduction in pain, relative to baseline.
Conclusions: The data presented here support the performance of a flexible CL-SCS system that can deliver a variety of waveforms, with amplitude programmed to patient comfort and automatically adjusted up to 50 times per second, to improve the consistency of therapy experience.
Registration: This study is registered on ClinicalTrials.gov number NCT05177354 https://clinicaltrials.gov/search? term=NCT05177354.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Academy of Pain Medicine.
Figures



References
-
- North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. 2007;61(2):361-368. discussion 368-9. 10.1227/01.NEU.0000255522.42579.EA - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

