Long-term Safety and Effectiveness of Cold-Crosslinked Hyaluronic Acid Fillers: Multicenter, Randomized, Controlled, Double-Blind Study
- PMID: 40378267
- PMCID: PMC12260371
- DOI: 10.1093/asj/sjaf080
Long-term Safety and Effectiveness of Cold-Crosslinked Hyaluronic Acid Fillers: Multicenter, Randomized, Controlled, Double-Blind Study
Abstract
Background: Evolysse Form (EVLF) and Evolysse Smooth (EVLS) (Symatese, Chaponost, France) are new hyaluronic acid fillers created using an innovative cold-crosslinking process.
Objectives: The authors of this study aim to collect safety and effectiveness data on new cold-crosslinked fillers to support the US approval for the correction of moderate-to-severe dynamic facial wrinkles and folds.
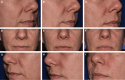
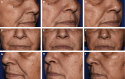
Methods: In this randomized, controlled, split-face study, 140 patients with moderate-to-severe nasolabial folds (NLFs) received a cold-crosslinked filler in 1 NLF (EVLF = 70, EVLS = 70) and a traditionally crosslinked filler, Restylane-L (RESL), in the contralateral fold and were followed through 12 months with an optional retreatment at that time point and subsequent 3 months of safety follow-up.
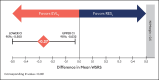
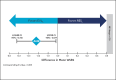
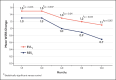
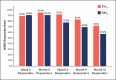
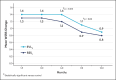
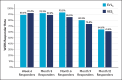
Results: The primary endpoint of mean Wrinkle Severity Rating Scale change from baseline to Month 6 as rated by the photographic review panel demonstrated noninferiority and statistical superiority for the cold-crosslinked fillers. Blinded evaluator Wrinkle Severity Rating Scale assessments showed a mean change from baseline that was statistically significantly better than RESL for EVLF at all visits through 12 months and for EVLS at 6 and 9 months. Most patients were responders on the Global Aesthetic Improvement Scale throughout the study, according to ratings by blinded evaluators, treating investigators, and patients. The FACE-Q appraisal of NLFs' overall mean score showed significant improvement from baseline (P < .0001) at all time points through Month 12 for all treatment groups. All treatments were well tolerated.
Conclusions: The new cold-crosslinked fillers were shown to be safe and effective for correction of NLFs, with results lasting for 1 year.
© The Author(s) 2025. Published by Oxford University Press on behalf of The Aesthetic Society.
Figures
References
-
- Klassen AF, Cano SJ, Scott AM, Pusic AL. Measuring outcomes that matter to face-lift patients: development and validation of FACE-Q appearance appraisal scales and adverse effects checklist for the lower face and neck. Plast Reconstr Surg. 2014;133:21–30. doi: 10.1097/01.prs.0000436814.11462.94 - DOI - PubMed
-
- Dayan S, Green JB, Schlesinger T, Dimitrijevic E, Chawla S, Sangha S. Higher responder rates observed with live participant assessment versus photographic assessment after VYC-20L hyaluronic acid treatment for chin augmentation. Aesthet Surg J. 2024;44:527–536. doi: 10.1093/asj/sjad348 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

