Inequalities in CAR T-cell therapy access for US patients with relapsed/refractory DLBCL: a SEER-Medicare data analysis
- PMID: 40378343
- PMCID: PMC12466227
- DOI: 10.1182/bloodadvances.2024015634
Inequalities in CAR T-cell therapy access for US patients with relapsed/refractory DLBCL: a SEER-Medicare data analysis
Abstract
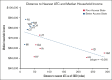
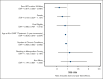
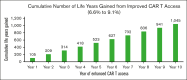
Chimeric antigen receptor (CAR) T-cell (CAR-T) therapy has shown curative potential for patients with diffuse large B-cell lymphoma (DLBCL) and other malignancies, but its accessibility among Medicare patients, particularly in disadvantaged populations, remains uncertain. This study aims to assess CAR-T use among Medicare patients with DLBCL receiving third-line or later (3L+) treatment, focusing on access disparities and their impact on clinical outcomes. Using Surveillance, Epidemiology, and End Results (SEER)-Medicare data from 2007 to 2020, multivariate logistic regression was used to evaluate patient characteristics and the effects of distance to authorized treatment centers (ATCs) on CAR-T access. Between 2017 and 2020, 2241 patients were treated for 3L+ DLBCL in the SEER-Medicare data, of whom 122 (5.4%) received CAR-Ts. CAR-T recipients were less likely to have multiple comorbidities (odds ratio [OR], 0.904; P = .001) but more likely to live in higher income areas (OR, 1.176; P = .004). If distance to the nearest ATC for "poor-access" states (average distance to ATC, 104.4 miles) decreased to the average distance in "better-access" states (34.2 miles), there would be a 37.6% increase in number of patients receiving CAR-Ts (6.6%-9.1%; P < .001). These findings highlight substantial disparities in CAR-T use, driven by geographic and socioeconomic factors. Addressing these barriers could significantly enhance equitable access to CAR-T therapy and improve outcomes for underserved populations, emphasizing the need for targeted interventions to reduce geographic and systemic barriers to care.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.P.C., J.T.S., and S.M. are employees of FTI Consulting, Inc, a consulting firm to health care, life sciences, government, and nongovernmental entities. S.V. and K.H., are employees of Kite/Gilead, the sponsor of this study. At the time of the study, A.R.P. was an employee of Kite/Gilead, the sponsor of this study. M.-A.P. reports honoraria from Adicet, Allogene, AlloVir, Caribou Biosciences, Celgene, Bristol Myers Squibb (BMS), Equilium, ExeVir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, Orca Bio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; serves on data safety monitoring boards (DSMBs) for Cidara Therapeutics and Sellas Life Sciences; serves on the scientific advisory board of NexImmune; reports ownership interests in NexImmune, Omeros, and Orca Bio; and has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. R.T.M. reports serving as consultant for Incyte, Kite/Gilead, and Novartis; receiving research support from Gamida Cell, Kite/Gilead, Orca Bio, and Novartis; serving on scientific advisory committee for Artiva; and participating in a DSMB for Novartis, Century Therapeutics, and Vor Biopharma. G.L.S. has received research funding to the institution from Janssen, Amgen, BMS, Beyond Spring, and GPCR Therapeutics, Inc; and is on the DSMB for ArcellX. L.C.A. is a consultant for Incyte, Kite/Gilead, and BMS.
Figures

References
-
- Leukemia and Lymphoma Society Facts and statistics overview. Updated 2023. https://www.lls.org/facts-and-statistics/facts-and-statistics-overview
-
- Ghilardi G, Williamson S, Pajarillo R, et al. CAR T-cell immunotherapy in minority patients with lymphoma. NEJM Evid. 2024;3(4) - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

