Status Epilepticus Protocol Variation Across Accredited National Association of Epilepsy Centers Members
- PMID: 40378375
- PMCID: PMC12089659
- DOI: 10.1212/WNL.0000000000213689
Status Epilepticus Protocol Variation Across Accredited National Association of Epilepsy Centers Members
Abstract
Objectives: Status epilepticus (SE) is a neurologic emergency that requires urgent recognition and medical management. SE management remains heterogeneous across centers.
Methods: We analyzed SE treatment protocols from level 3 and level 4 epilepsy centers. Discrete data including stabilization measures, timing of treatment phases, medications, doses, and routes of administration were collected from each protocol and described using frequency for categorical variables and median for continuous variables. The distribution of treatment times and dosing were compared with the AES guideline.
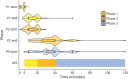
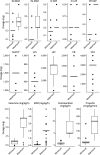
Results: A total of 256 SE treatment protocols were included. Only 66% of SE protocols detailed treatment times. Doses below recommendations occurred in 4% of protocols for initial benzodiazepine (BZD) and 14% for first non-BZD medications. Infusion therapy was outlined in 61% of protocols.
Discussion: Despite the importance of timeliness in SE management, one third of institutional protocols did not specify treatment times. This analysis of US hospital inpatient SE protocols provides expert opinion regarding infusion therapy management and highlights gaps and targets for improvement in SE treatment.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures
References
-
- Silbergleit R, Lowenstein D, Durkalski V, Conwit R, Neurological Emergency Treatment Trials NETT Investigators. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011;52(suppl 8):45-47. doi:10.1111/j.1528-1167.2011.03235.x - DOI - PMC - PubMed
-
- Chamberlain JM, Kapur J, Shinnar S, et al. . Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395(10231):1217-1224. doi:10.1016/S0140-6736(20)30611-5 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
