Impact of extending the original criteria in the Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study (CROSS) regimen on treatment outcome in locally advanced esophageal cancer patients
- PMID: 40378527
- PMCID: PMC12145669
- DOI: 10.1016/j.esmoop.2025.105098
Impact of extending the original criteria in the Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study (CROSS) regimen on treatment outcome in locally advanced esophageal cancer patients
Abstract
Background: The Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study (CROSS) regimen is currently offered to locally advanced esophageal cancer patients beyond the original eligibility criteria. This national population-based study assessed the safety in implementation regarding treatment outcome when extending these criteria.
Patients and methods: Locally advanced esophageal cancer (cT1N+/T2-4aN0-3/M0) patients (n = 5061) from the Netherlands Cancer Registry treated according to the neoadjuvant chemoradiotherapy (nCRT) CROSS regimen between 2015 and 2022 were analyzed. A total of 1958 complied with the original criteria (O-CROSS group) and 1348 with one or more extended criteria (tumor length >8 cm, age >75 years, WHO score >2 and/or weight loss >10%) (E-CROSS group), eventually followed by resection in 1342 O-CROSS patients and 852 E-CROSS patients. Primary outcome was overall survival (OS), i.e. time interval from onset of nCRT (OS-nCRT) and from date of surgery (OS-surgery) until death or last follow-up. Secondary outcomes were disease-free survival, pathological complete response (pCR), surgical radicality, post-operative morbidity and mortality. Data were analyzed using the Kaplan-Meier method and Cox proportional hazards models.
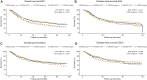
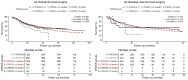
Results: OS-nCRT was significantly lower in the E-CROSS compared with the O-CROSS (median of 30.3 months, 95% confidence interval 27.2-33.5 months versus 45.9 months, 95% CI 38.4-53.4 months, P < 0.001). Similarly, differences were observed in OS-surgery. When OS-nCRT and OS-surgery were adjusted for baseline covariates, however, no difference was found between both groups. Moreover, no differences were observed in disease-free survival, surgical radicality, and pCR. While not affecting post-operative mortality, significantly more anastomotic leakages and thromboembolic post-operative complications were seen in the O-CROSS group.
Conclusion: Extending the CROSS criteria was associated with lower OS, which was caused by the higher age, weight loss >10% and WHO score in the E-CROSS group. The CROSS regimen can be used in a 'real-world' setting but individual factors that may contribute to OS should be considered in decision-making.
Keywords: esophageal carcinoma; neoadjuvant chemoradiotherapy; pathological response; surgery; survival; treatment outcome.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Shapiro J., van Lanschot J.J.B., Hulshof M.C.C.M., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. - PubMed
-
- Grabsch HI, Mapstone NP, Novelli M. Dataset for histopathological reporting of oesophageal and gastric carcinoma October 2019. R Coll Pathol. 2019;3:1–58.
-
- Bertero L., Massa F., Metovic J., et al. Eighth Edition of the UICC Classification of Malignant Tumours: an overview of the changes in the pathological TNM classification criteria—what has changed and why? Virchows Arch. 2018;472(4):519–531. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

