Tumor location as a risk factor for severe immune-related adverse events
- PMID: 40379269
- PMCID: PMC12083421
- DOI: 10.1136/jitc-2024-011312
Tumor location as a risk factor for severe immune-related adverse events
Abstract
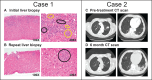
Immune-related adverse events (irAEs) can cause severe morbidity and mortality, and they impair treatment with immune checkpoint inhibitors (ICI). Risk factors for irAEs are not well understood.We observed cases of patients having tumor deposits in their liver and lung during a workup of irAEs, which led us to hypothesize that the presence of tumor in an organ would increase the odds of developing severe irAEs in that organ. We then performed a retrospective cohort study that included patients who received an ICI for the treatment of cancer and were hospitalized between February 2011 and November 2021 at the Massachusetts General Hospital.We reviewed 384 patients hospitalized with concern for any irAE. A clinical diagnosis of ICI-related hepatitis occurred in 18% of patients with liver tumor deposits versus 8% of those without (OR 2.23, 95% CI (1.10 to 4.43), p=0.02). ICI-related pneumonitis occurred in 10% of patients with lung tumor deposits versus 4.4% of those without (OR 2.45, 95% CI (1.06 to 6.36), p=0.047). A combined analysis for liver and lung lesions demonstrated that the presence of tumor deposits in an organ increased the odds of having an irAE in that organ by over twofold (OR 2.31, 95% CI (1.34 to 3.99), p=0.002).Our results suggest that the presence of tumor deposits may represent a novel risk factor for severe irAEs in that organ.
Keywords: Biomarker; Immune Checkpoint Inhibitor; Immune related adverse event - irAE; Immunotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: The authors would like to disclose the following financial conflicts, which do not directly relate to this work. SMB: consultant to Two River Consulting, Third Rock Ventures; and equity in Kronos Bio, 76Bio, Allogene Therapeutics, and Candid Therapeutics. BO: IP and royalties from Tissium. LZ: consultant to Bristol Myers Squibb, Merck. MMK: consultant to AstraZeneca, Pfizer, Repare, Boehringer Ingelheim, Sanofi, AbbVie, Daiichi-Sankyo, BMS, Roche; and royalties from Elsevier. A-CV: consultant to Bristol Myers Squibb; and financial interest in 10X Genomics. KLR: advisory board to SAGA Diagnostics; advisory board to Regeneron, speaker’s fees from CMEOutfitters, Medscape; and research funding from Bristol Myers Squibb. RJS: consultant to Bristol Myers Squibb, Merck, Pfizer, Marengo Therapeutics, Novartis, Eisai, Iovance, OncoSec, AstraZeneca; and research funding from Merck.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
