Effect of a smart ring-assisted physiotherapeutic intervention on the postoperative outcomes 12 months after a total knee replacement (SmarTKRing): protocol for a randomised controlled trial
- PMID: 40379331
- PMCID: PMC12083359
- DOI: 10.1136/bmjopen-2024-091956
Effect of a smart ring-assisted physiotherapeutic intervention on the postoperative outcomes 12 months after a total knee replacement (SmarTKRing): protocol for a randomised controlled trial
Abstract
Introduction: This protocol assesses the effect of a wearable activity tracker-assisted physiotherapeutic intervention on postoperative outcomes in patients undergoing total knee replacement (TKR) surgery. Despite advancements in TKR technology, patient dissatisfaction remains a concern, with adherence to physical activity guidelines being particularly poor among those with knee osteoarthritis. The primary aim of this trial is to evaluate the effectiveness of a smart ring-assisted physiotherapeutic intervention in improving outcomes, as measured by the Oxford Knee Score (OKS), 12 months after a TKR.
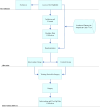
Methods and analysis: We are conducting this randomised controlled trial at Coxa Hospital for Joint Replacement in Finland, where participants are randomly assigned to either an intervention with smart ring-supported physiotherapeutic intervention or a usual care control group in a 1:1 ratio in patients undergoing TKR surgery. The primary outcome measure is the OKS at 12 months after surgery. Secondary outcomes encompass OKS variance, poor postoperative outcome (defined as ≤7 points change on the OKS), quality of life questionnaire EuroQol 5-Dimensions 5-Levels(EQ-5D-5L), knee range of motion, pain scores, patient satisfaction and healthcare resource utilisation. The study population comprises patients aged 18-70 undergoing unilateral primary TKR for knee osteoarthritis. Statistical analysis involves logistic regression and linear mixed models to assess group differences in outcomes over time, with adjustments for relevant covariates.
Ethics and dissemination: The trial was approved by the Tampere University Hospital Ethical Committee (R22078) and participants are required to provide written informed consent. Procedures in conducting the trial are aligned with the principles of Good Scientific Practice outlined by the Finnish Advisory Board on Research Integrity. The results of this trial will be disseminated as a series of articles published in a peer-reviewed medical journal.
Trial registration number: NCT05599776.
Keywords: Knee; ORTHOPAEDIC & TRAUMA SURGERY; Orthopedics; Patients; Randomized Controlled Trial; Wearable Electronic Devices.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Bone & Joint Predicting dissatisfaction following total knee replacement. [21-Apr-2024]. https://boneandjoint.org.uk/Article/10.1302/0301-620X.92B9.24394 Available. Accessed. - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
