Mindfulness-based cognitive therapy versus treatment as usual after non-remission with NHS Talking Therapies high-intensity psychological therapy for depression: a UK-based clinical effectiveness and cost-effectiveness randomised, controlled, superiority trial
- PMID: 40379363
- PMCID: PMC12078190
- DOI: 10.1016/S2215-0366(25)00105-1
Mindfulness-based cognitive therapy versus treatment as usual after non-remission with NHS Talking Therapies high-intensity psychological therapy for depression: a UK-based clinical effectiveness and cost-effectiveness randomised, controlled, superiority trial
Abstract
Background: Non-remission after psychological therapy for major depressive disorder is common, yet there are no established further-line treatments. In the UK National Health Service (NHS) Talking Therapies programme, about 50% of patients with depression who come to the end of the stepped care pathway do not show remission of symptoms. We aimed to investigate whether mindfulness-based cognitive therapy (MBCT) can improve clinical outcomes and whether the additional financial cost is worthwhile.
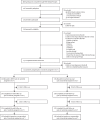
Methods: We conducted a parallel, randomised, controlled, superiority trial in three sites in the UK (Devon, London, and Sussex). Patients with current major depressive disorder whose symptoms had not reached remission (assessed as Patient Health Questionnaire-9 [PHQ-9] score ≥10) after an adequate dose of NHS Talking Therapies high-intensity therapy (≥12 sessions) were recruited from 20 NHS Talking Therapies services. Participants were allocated through remote random assignment (1:1) to MBCT plus treatment as usual or treatment as usual alone at the UK Clinical Research Collaboration-registered Exeter Clinical Trials Unit with minimisation on depression severity (PHQ-9 score <19 vs ≥19), antidepressant use at baseline (yes vs no), and recruitment site (Devon vs London vs Sussex). MBCT was delivered via videoconference and comprised an individual orientation session and eight weekly group sessions. The primary clinical outcome was reduction in depression symptomatology at 34 weeks after randomisation, using the PHQ-9. Cost-effectiveness was evaluated in terms of costs to primary, secondary, and tertiary health and social care services collected using the Adult Service Use Schedule and quality-adjusted life-years (QALYs) via health utilities derived from the EQ-5D. Primary outcome analyses were masked in the intention-to-treat population using observed data only. Lived experience experts were integral to all stages of this research. The trial was prospectively registered with ISRCTN, ISRCTN17755571.
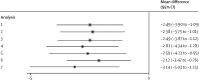
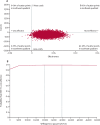
Findings: Between April 20, 2021, and Jan 24, 2023, we enrolled 234 eligible participants, 166 (71%) of whom identified as women, 65 (28%) as men, one (<1%) as other, and two (1%) preferred not to say. The mean age was 42·5 years (SD 13·9). 201 (86%) of 234 participants were White. 118 participants were assigned to MBCT plus treatment as usual and 116 to treatment as usual alone, 101 and 102 of whom completed the final follow-up, respectively. At 34 weeks after randomisation, the MBCT plus treatment as usual group had significantly lower levels of depression symptomatology than the treatment as usual alone group (adjusted between-group difference -2·49, 95% CI -3·89 to -1·09; p=0·0006; Cohen's d -0·41, 95% CI -0·67 to -0·15). Utility scores were higher and costs were lower in the MBCT group (adjusted mean cost difference -£245·23, 95% CI -581·92 to 91·46; p=0·15) over the course of the study. The MBCT plus treatment as usual group had an estimated 99% chance of being cost-effective at the £20 000 per QALY threshold. Bootstrapped mean differences in costs and QALYs indicated a 91% probability of MBCT plus treatment as usual being less costly and more effective than treatment as usual alone for all values a decision maker might be willing to pay for an improvement in QALYs. We observed no trial or treatment-related serious adverse events and no other evidence of harms.
Interpretation: Our findings show that mindfulness-based treatment can be beneficial after non-remission from major depressive disorder following psychological, stepped care treatments. Together with evidence from previous studies of non-remission after pharmacological treatment, our findings establish MBCT, an easily scalable group-based intervention, as a further-line treatment. Implementation of MBCT for patients who continue to have major depressive disorder in routine care settings (NHS Talking Therapies and beyond) is warranted.
Funding: UK National Institute for Health and Care Research Research for Patient Benefit programme.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests TB is the author of a book on mindfulness-based cognitive therapy (MBCT). TB, CS, and FAR regularly provide workshops on mindfulness-based interventions. CS is a member of a training organisation that has been commissioned by NHS England to deliver MBCT training across NHS Talking Therapies services in England and co-leads the Sussex Mindfulness Centre. TB and CS are co-investigators of a programme grant evaluating an adapted MBCT course for adolescents experiencing depression. CS is co-investigator on a grant evaluating an adapted MBCT course for NHS staff. BDD is the lead of the University of Exeter AccEPT clinic, which offers courses of MBCT. AHY serves as principal investigator for several psychopharmacological trials for treatment-resistant depression and has provided paid lectures and participated on advisory boards for Flow Neuroscience, Novartis, Roche, Janssen, Takeda, Noema Pharma, Compass, Astrazenaca, Boehringer Ingelheim, Eli Lilly, LivaNova, Lundbeck, Sunovion, Servier, Janssen, Allegan, Bionomics, Sumitomo Dainippon Pharma, Sage, and Neurocentrx. All other authors declare no competing interests.
Figures
References
-
- Amos TB, Tandon N, Lefebvre P, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. J Clin Psychiatry. 2018;79:24–32. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

