SLE-DAS enables an accurate definition of severe lupus disease activity: derivation and validation in a post hoc study of anifrolumab phase II and III studies
- PMID: 40379439
- PMCID: PMC12086896
- DOI: 10.1136/lupus-2025-001499
SLE-DAS enables an accurate definition of severe lupus disease activity: derivation and validation in a post hoc study of anifrolumab phase II and III studies
Abstract
Objectives: This study aimed to derive and validate a cut-off for severe disease activity (SDA) using the SLE Disease Activity Score (SLE-DAS) and compare its accuracy and impact on health-related quality of life (HR-QoL) with the British Isles Lupus Assessment Group 2004 (BILAG-2004) and SLE Disease Activity Index 2000 (SLEDAI-2K).
Methods: We performed a post hoc analysis of pooled placebo arm data from the MUSE (A Phase II, Randomized Study to Evaluate the Efficacy and Safety of MEDI-546 in Subjects with Systemic Lupus Erythematosus), TULIP-1 and TULIP-2 (Treatment of Uncontrolled Lupus via the Interferon Pathway) trials, including 438 patients with moderate-to-severe SLE. SLE-DAS was scored retrospectively, and a cut-off for SDA was derived using receiver operating characteristic (ROC) curves against the BILAG-2004 numerical score >11 as gold standard. Multiple linear regression analysis and Cohen's d effect size were applied to evaluate the effectiveness of SLE-DAS, BILAG-2004 and SLEDAI-2K SDA classifications in capturing HR-QoL patient-reported outcomes (PROs).
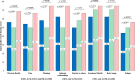
Results: The optimal SLE-DAS cut-off for SDA was >9.90 (area under the ROC curve=0.847, sensitivity=77.8%, specificity=79.6%). Patients classified as SDA by both SLE-DAS and BILAG-2004 or only by SLE-DAS exhibited similar disease activity, while those classified by BILAG-2004 alone had less severe disease and better HR-QoL. The SLE-DAS cut-off was associated with worse HR-QoL across multiple PROs more consistently than BILAG-2004 or SLEDAI-2K.
Conclusion: The SLE-DAS cut-off for SDA provides an accurate definition of SDA in SLE, with good discriminative power and consistent associations with worse HR-QoL. This SLE-DAS definition enhances disease activity classification and offers a practical tool for guiding treatment decisions in clinical practice, as well as selecting patients with SDA for inclusion in clinical trials.
Keywords: Health-Related Quality Of Life; Outcomes research; Systemic Lupus Erythematosus.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Yee C-S, Farewell VT, Isenberg DA, et al. The use of Systemic Lupus Erythematosus Disease Activity Index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology (Oxford) 2011;50:982–8. doi: 10.1093/rheumatology/keq376. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
