AxoDen: An Algorithm for the Automated Quantification of Axonal Density in Defined Brain Regions
- PMID: 40379480
- PMCID: PMC12135899
- DOI: 10.1523/ENEURO.0233-24.2025
AxoDen: An Algorithm for the Automated Quantification of Axonal Density in Defined Brain Regions
Abstract
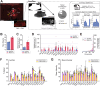
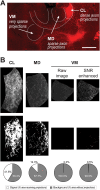
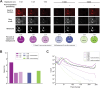
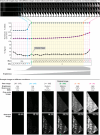
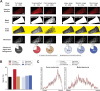
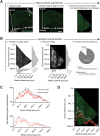
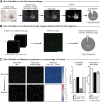
The rodent brain contains 70,000,000+ neurons interconnected via complex axonal circuits with varying architectures. Neural pathologies are often associated with anatomical changes in these axonal projections and synaptic connections. Notably, axonal density variations of local and long-range projections increase or decrease as a function of the strengthening or weakening, respectively, of the information flow between brain regions. Traditionally, histological quantification of axonal inputs relied on assessing the fluorescence intensity in the brain region of interest. Despite yielding valuable insights, this conventional method is notably susceptible to background fluorescence, postacquisition adjustments, and inter-researcher variability. Additionally, it fails to account for nonuniform innervation across brain regions, thus overlooking critical data such as innervation percentages and axonal distribution patterns. In response to these challenges, we introduce AxoDen, an open-source semiautomated platform designed to increase the speed and rigor of axon quantifications for basic neuroscience discovery. AxoDen processes user-defined brain regions of interests incorporating dynamic thresholding of grayscale-transformed images to facilitate binarized pixel measurements. Here, in mice, we show that AxoDen segregates the image content into signal and nonsignal categories, effectively eliminating background interference and enabling the exclusive measurement of fluorescence from axonal projections. AxoDen provides detailed and accurate representations of axonal density and spatial distribution. AxoDen's advanced yet user-friendly platform enhances the reliability and efficiency of axonal density analysis and facilitates access to unbiased high-quality data analysis with no technical background or coding experience required. AxoDen is ad libitum available to everyone as a valuable neuroscience tool for dissecting axonal innervation patterns in precisely defined brain regions.
Keywords: axon; imaging; microscopy; neuron; open-source; toolbox.
Copyright © 2025 Sandoval Ortega et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
